|
Manduca sexta Caterpillars Exhibit Rhythms in timeless Gene Expression at Different Times of Day Rowan Cain, Subeen Lee, Matthew Quintanilla, Kylie Rankin, Howard Community College Mentored by: Ellena McCarthy, PhD |
Abstract
Manduca sexta, known as the tobacco hornworm, is a common pest of Solanaceous plants, therefore making the molecular mechanisms influencing Manduca sexta’s feeding behavior a topic of interest. Circadian rhythms, 24-hour internally driven biological rhythms, are directly involved in the timing and types of many organism’s behaviors, like the feeding observed in certain caterpillars. Adult Manduca sexta have a functioning circadian clock that influences their behavior, but whether it is functional in larval caterpillars is still unknown. In this study, we sought to determine whether the 5th instar larvae of Manduca sexta exhibit rhythmic clock gene expression, by measuring expression of the clock gene, timeless (tim). Gut tissues of 5th instar Manduca sexta larvae were harvested at different times of the day, ZT3 and ZT15, and tested for rhythmic expression of Tim. A statistically significant difference was found in the relative expression of Tim at these two ZT times. This data is consistent with the larvae having a functional circadian clock.
Introduction
Most living organisms have evolved an endogenous physiological clock driven by 24-hour oscillating molecular mechanisms. This internal timekeeping mechanism regulates many behavioral processes, such as sleep/wake and feed/fast cycles. These rhythms are the products of the circadian clock. Circadian, of Latin origin “circa diem,” meaning “about a day.” Circadian rhythms are endogenous (internally driven), meaning they will persist even without environmental stimuli, such as the light/dark cycle induced by the sun [1].
Circadian rhythms are driven by molecular clocks in individual cells. These molecular clocks are composed of transcriptional/translational feedback loops that utilize positive elements to promote transcription and negative elements that, when translated, inhibit the activators [2]. In organisms, such as the adult Manduca sexta moth, two positive transcription factors called CLOCK and CYCLE activate the expression of period (per) and timeless (tim) genes. Once expressed, these genes are translated into their respective proteins, PER, and TIM, which accumulate in the cytoplasm of the cell before combining (as a heterodimer) and returning to the nucleus to repress their own transcription [3]. Environmental cues, known as zeitgebers, can reset the circadian rhythm. The light cycle is the most pervasive and strong zeitgeber, but many other factors can influence a circadian cycle, including temperature, activity, and the presence of food. The circadian clock can be synchronized to an altered or different zeitgeber [4].
The larval form of Manduca sexta, the tobacco hornworm, is a common pest of Solanaceous plants, including tobacco and tomato crops. These pests can cause significant economic damage and decimate crops through ravenous feeding during late instars when the larvae are preparing for pupation [5]. Other caterpillars have been shown to exhibit rhythms in feeding activity, such as feeding during the night, and resting during the day [6]. If tobacco hornworms have a functional circadian clock, their internal clock could contribute to the timing of feeding and digestion activity. The tobacco hornworm is a model organism and is often used in research concerning biological processes. Its small size, short life cycle, and extensive knowledge of its genome make it an ideal candidate for research. Moreover, understanding the feeding behaviors and rhythms of these insects is of great significance to the agricultural industry: by developing an in-depth understanding of possible circadian rhythms in the tobacco hornworm, agriculturalists can better combat the larvae’s destructive nature.
Previous research has shown that many insects possess a functional molecular circadian clock [7]. Much less is known about circadian rhythms in larval moths, with some larval species exhibiting behavior that isn’t explicitly nocturnal or diurnal, with slight rhythmic behaviors often being driven by external cues [8] [9]. It is known, however, that in some insects, the transcription of clock genes, like per and tim, is driven by the CLK/CYC PER/TIM feedback loop [10] In 24 hours, the transcription factors CLOCK and CYCLE bond to the response element E-box, causing per and tim to be expressed (Figure 1) [10]. Once expressed, PERIOD (PER) and (TIM) form heterodimers and return to the nucleus, thus repressing their transcription [10]. Concurrently, other genes with the E-box response element, have downstream effects on many rhythmic behaviors [10].
Adult Manduca sexta, a member of the Hawkmoth family, displays rhythmic locomotor behavior: in light-dark (LD) conditions, the moths display rhythmic flight restricted to the dark part of the day [11] [12]. In dark-dark (DD) conditions they maintain 24-hour rhythmic flight patterns [12]. The hawk moth’s circadian clock holds influence over behavioral responses, such as floral odor signals [12]. This response is activated within the olfactory neurons in adult hawk moth antennae and shows 24-hour oscillations in responsiveness during LL, DD, and 12L:12D conditions [12]. While it is known that adults of Manduca sexta display circadian rhythms in behavior driven by a functional circadian clock, it is not yet known if these rhythms are present in larval development, even though they possess the needed genes in their genome.
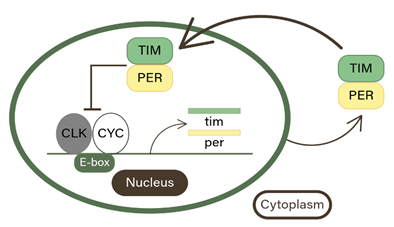
Figure 1. The Circadian Transcription/Translation Feedback Loop. PER and TIM dimerize to undergo a negative transcription-translation feedback loop that induces and inhibits their own gene expression in the nucleus of the cell in an approximately 24-hour cycle.
Recently, Manduca sexta larvae have been found to exhibit rhythmic feeding behavior in the 5th instar of development, with data suggesting rhythmic expression of the per gene that could coincide with circadian rhythms [13]. However, the analysis was performed on a very small sample size, leading to the evidence on rhythmic gene expression being inconclusive. We sought to expand on this research to determine if larval Manduca sexta exhibits rhythmic gene expression by determining if Tim gene expression fluctuates throughout 24 hours. We did this by measuring and analyzing Tim expression levels in gut tissue samples collected at two different Zeitgeber times ZT3 & ZT15 using quantitative polymerase chain reaction (qPCR). RNA was extracted from samples and then converted into cDNA using the enzyme reverse transcriptase. This cDNA was then used as a template for the qPCR reaction. We then analyzed Tim expression levels through their amplification and determined if they fluctuate at different times of day, exhibiting rhythmicity.
Methods
Caterpillar husbandry
As described previously in Waters et al. 2021 [13], the Manduca sexta eggs were sourced from Carolina Biological Supplies and raised in lidded petri dishes with temperatures ranging from 22-24℃. The caterpillars’ health and instar were monitored regularly, and they were fed the leaves of a fertilized tomato plant. Throughout their lifespan, the caterpillars were kept on a 16:8 ZT cycle, which replicated summer conditions of 16 hours of light and 8 hours of darkness. The time regime was implemented using a time and the light/dark transition was bimodal, on or off. Once the caterpillars reached their fifth instar larval stage, their head and gut tissues were collected at ZT 3, three hours after the lights came on, and at ZT 15, one hour before the lights were turned off.
Tissue harvesting
Tissue harvesting procedures were performed as described in Waters et al [13]. After behavioral testing was completed, the caterpillars were placed in an ice bath for 15 minutes for anesthetization. Once anesthetization was complete, the caterpillars were dissected with dissection kits sterilized with 95% ethanol and RNase-zap wipes. The RNase-zap wipes aimed to prevent RNase or other enzymes from contaminating the harvested tissue samples. To extract midgut samples, an incision was made from the posterior region of the prolegs, up to the midsection of the abdominal region. A small portion of the midgut was extracted from the caterpillars and suspended in RNAlater (Thermo Fisher) to preserve the stability of the mRNA.
These samples were then refrigerated in RNAlater at 4℃ for a night, then stored at -80℃ until RNA extraction was performed.
RNA Extraction
RNA extraction was performed using a RNeasy Kit (Qiagen), as detailed in the RNeasy Mini Kit manual. The only deviation was that genomic DNA was removed using a gDNA spin column. After extraction, RNA quantity and purity were assessed. Measurements were performed using the NanoDrop spectrophotometer, with a focus on the 260/280 ratio. The numbers 260 and 280 indicate the wavelengths of light in nanometers used for absorbance measurements. The 260/280 ratio assesses the purity of RNA from DNA and proteins, with an optimal range of 2.0-2.2 for RNA.
cDNA Synthesis
To perform cDNA Synthesis on our extracted RNA samples, RevertAid First Strand cDNA Synthesis Kit (Fisher) was used according to the manufacturer’s instructions. To confirm the functionality of amplification, a positive control well included substantia Glyceraldehyde-3-phosphate dehydrogenase (Gapdh) RNA, while the negative control well contained template RNA but no reverse transcriptase. The sample then went into the thermocycle for 60 minutes at 42℃ and then 10 minutes at 70℃. After cDNA Synthesis, the cDNA samples were treated with RNase H to degrade the remaining RNA in the sample before running qPCR. The samples were incubated at 37℃ for 20 minutes.
Validating Primers and cDNA Synthesis
To carry out qPCR, primer sets targeted to our genes of interest were needed for amplification. The sequences for primer sets used are as follows: Actin F 5’ CGTGCCCATCTACGAAGGTT 3’, Actin R 5’ CTGCTCGAAGTCAAGAGCGA 3’, Timeless F 5’ GCTGCTCAGGAATATCTTGCAT 3’, Timeless R 5’ GGATCTGGTTTTGTACGGTGTG 3’, Period F 5′ CCAAGGAGCAGAAGTACAGGAG 3′, Period R 5′ GCTACCTCAGCGGGTTTAGT 3′. The actin and per primer sets were designed and tested by previous URSC students [14]. The tim primer set was previously published [15].
To ensure the specificity of the primer sets, they were validated by performing polymerase chain reaction on caterpillar cDNA and then analyzing the product size using gel electrophoresis. The expected product for each primer set was actin 190 base pairs (bp), tim 92 bp, and per 73 bp. The products were sized by comparing our results to a 100 bp DNA ladder (NEB). Each primer yielded a single band at the expected size.
Quantitative Polymerase Chain Reaction (qPCR)
qPCR was performed on caterpillar cDNA samples, following the protocol described in the SsoAdvanced Universal SYBR Green Supermix kit (Bio-Rad). Reactions were performed in triplicate, meaning that every caterpillar sample was amplified six times. For each caterpillar sample, three replicate reactions were performed using primers for actin, the reference gene and three replicate reactions were performed using primers for our gene of interest, tim. Gapdh cDNA, generated using the RevertAid First Strand cDNA Synthesis Kit (Fisher), was used as a positive control to ensure the cDNA synthesis had been successful, while reactions without the cDNA template served as a no-template control (NTC).
Amplification was quantified by assigning each sample a Cq, or quantification cycle, which is a value that describes at which cycle the amplification crossed an arbitrary threshold. Following qPCR, we determined if the data was viable by ensuring that the usable sample’s triplicate reaction had amplification that occurred within 0.5-1 cycles within one another, and that no amplification of the negative controls had occurred before 35 cycles. If the data was viable, we took the average of the three Cq values for each triplicate, excluding outliers. We then calculated the delta Cq value by subtracting the average Tim Cq value from the corresponding Actin Cq value of each caterpillar. We used these values to determine if there was a significant difference in gene expression at the two ZT times.
Statistical Analysis
To analyze the relationship between caterpillar feeding behavior and qPCR data, analytics were viewed through the R software provided by the R Project Core Team. Normality was first tested through the Shapiro-Wilk normality test. Then, the homogeneity of variance was analyzed through Bartlet’s test. Since the data was found to be normal and the variance to be homogeneous, the qPCR results for ZT3 and ZT15 caterpillars were compared through the Welch Two Sample T-test, which utilized ΔCT values to determine whether tim expression varied at different times of the day. To determine whether the values presented held statistical significance in the t-test, p-values below 0.05 were deemed significant.
Results
To investigate Tim expressions at different times of the day, qPCR analyses were carried out on midgut samples harvested at ZT3 (n=16) and ZT15 (n=11) by the previous research team in Spring 2022. Due to the number of samples and replicates needed, multiple runs of qPCR were done. SYBR Green Fluorescence graphs were used to visualize expression levels.
To validate the proper functioning of the quantitative PCR procedure, positive and negative control runs were performed (Figure 2). (Gapdh) was used as a positive control in the qPCR analysis. Early Gapdh amplification was expected due to high mRNA concentration initially. Rapid Gapdh amplification confirmed that our cDNA synthesis was robust. The Non-Template Control (NTC), in which no cDNA template was added, demonstrated an absence of amplification as expected. As a negative control, the NTC effectively ruled out potential contamination or false-positive signals, highlighting the robustness of our experimental setup. The successful performance of Gapdh as a positive control and the absence of amplification in NTC replicates validate the reliability of our reverse transcription and qPCR processes.
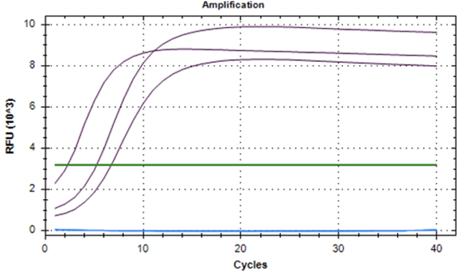
Figure 2: Representative qPCR traces for Gapdh and for the Non-Template Control. The CT threshold (green) was placed at 3×103 RFU as a horizontal line for calculating CT values. The appearance of the Gapdh (purple) is rapid, attributed to a high concentration of mRNA and a robust cDNA synthesis process. The non-template controls (light blue) maintain a flat line at 0 relative fluorescence units (RFU), showing no amplification, as expected.
To assess the relative levels of Tim expression at different times of day, we performed qPCR on the caterpillar midgut samples harvested at ZT 3 (n=16) and ZT15 (n=11) for expression of Tim and for our reference gene, Actin. Each reaction was performed in triplicate. Representative qPCR traces for a ZT 3 caterpillar sample and for a ZT15 caterpillar sample are shown in Figure 3 and 4, respectively. For our samples at ZT3, Actin amplified between 16-21 cycles, while the Tim samples amplified at between 20-25 cycles. For ZT15, Actin was found to amplify between 16-23 cycles, whereas Tim amplified between 23-28 cycles. For each trace, the cycle threshold (CT), which is the number of cycles necessary to reach the threshold, was determined. The mean CT for Tim expression of each sample and the mean CT for Actin of each sample was calculated.
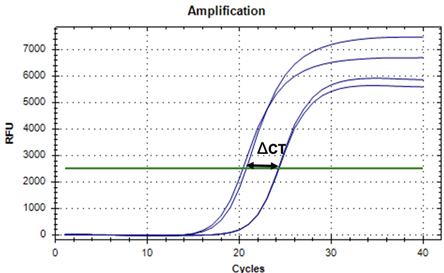
Figure 3: Representative qPCR trace for Tim expression at ZT3. The Cycle Threshold, represented by a horizontal green line, indicates the threshold for calculating CT values. In this trace graph, the traces for the reference gene, Actin, are on the left. The traces for our target gene, tim, are on the right. Actin is more abundant and therefore amplifies first on trace graphs. Representative traces were taken from Caterpillar #18 at ZT3. Relative Tim expression was assessed by calculating the delta CT (ΔCT) value for each sample. ΔCT represents the difference in cycles it takes for Actin and Tim to amplify. The smaller ΔCT value, at that given time, portrays higher gene expression.
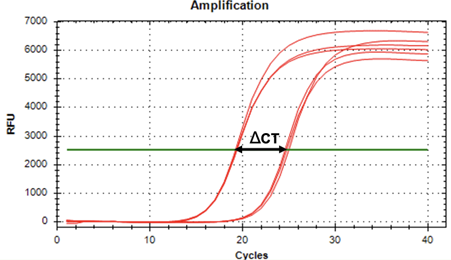
Figure 4: Representative qPCR traces for Tim expression at ZT15. In this graph, one sees the reference gene is Actin (seen amplifying on the left), and the target gene is tim (seen amplifying on the right). Representative traces were taken from caterpillar #30 at ZT15, which was chosen due to being within 0.5 cycles of the mean ΔCT value of 5.88±0.56 for ZT15. The larger ΔCT value for caterpillars at ZT15 indicates lower Tim gene expression than seen at ZT3.
Once all runs of qPCR were checked to ensure that the controls behaved as expected and all triplicate runs amplified similarly, meaning the CT values were within one cycle of one another, we calculated the delta CT (ΔCT) value for each ZT time by subtracting when the Tim traces crossed the cycle threshold from when Actin traces crossed the cycle threshold. ΔCT represents the difference in expression levels for Actin and Tim, quantifying relative gene expression. The average ΔCT for ZT3 was found to be 4.25 ± 0.45, and the average ΔCT for ZT15 was 5.88 ±0.56 (Figure 5). This difference was found to be statistically significant (p<0.05). A lower ΔCT value was found for ZT3, indicating a higher level of expression of Tim earlier in the day. The higher ΔCT value seen at ZT15 indicates lower Tim expression later in the day. This difference in expression indicates rhythmicity in the Tim expression for larval Manduca sexta.
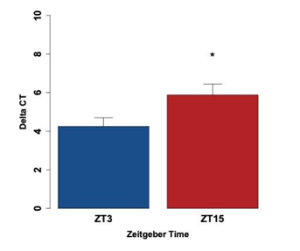
Figure 5: Bar graph displaying the mean ΔCT values for ZT3 (blue) and ZT15 (red) caterpillar midgut samples. The difference in mean ΔCT values between ZT3 and ZT15 caterpillars was found to be statistically significant. The star indicates statistical significance, p<0.05. Higher gene expression is indicated by a smaller ΔCT value. The error bars represent the standard error of the mean.
Discussion
Previous studies have shown that adult Manduca sexta displays rhythmic locomotor behavior that corresponds with circadian gene expression of per and tim. Highlighting the role of the per gene, previous research conducted on fifth instar Manduca sexta larvae observed rhythmicity in Per expression but yielded promising, but inconclusive results due to a small sample size [13]. To supplement this data, our experiment featured a larger sample size, and a greater focus on the expression of the tim gene. The Zeitgeber times, ZT3 and ZT15, analyzed in our study were chosen by a previous cohort to analyze the potential rhythms in behavior and gene expression. These time points were chosen twelve hours apart so as to maximize likelihood of observing rhythmic activity if it was present. These two timepoints in the early morning and late afternoon were also chosen because they were during the light period because testing and manipulating the caterpillars in darkness posed significant technical challenges.
Since PER and TIM proteins dimerize [2], we hypothesized that analyzing Tim would yield results that could coincide with levels of Per expression described in Waters et al. In our experiment, we found the first evidence that the Tim gene is rhythmically expressed in the fifth-instar larvae of Manduca sexta, which could be a possible indication of a functioning circadian clock in this stage of larval development.
Most caterpillars exhibit nocturnal feeding behavior to avoid predation [6], such as Oleria baizana, of the same order as Manduca sexta, described climbing plants to feed at night [16]. While the analysis of behavioral characteristics (such as a nocturnal or diurnal nature) lays the groundwork for an understanding of sleep/wake and eat/fast cycles, incorporating a molecular basis through which these behaviors can be predicted allows agricultural specialists to better combat the larvae’s destructive nature as a pest, such as by informing farmers of when the best times are to apply pesticides to reduce Manduca sexta’s consumption of crops. As behaviors such as feeding are subject to synchronization with external cues, an internal clock would provide a passive conduit through which researchers can understand behavior.
Adult Manduca sexta moths have been shown to exhibit nocturnal locomotor behavior and feeding behavior [11], which is expected for moth species. However, Waters et al. describe that the Manduca sexta larval feeding behavior that they observed was most prevalent during the early day [13]. It is interesting to note that both the adult and larvae likely exhibit rhythmic feeding, but the peak of that feeding behavior is at a different time of the circadian cycle. The peak of adult feeding behavior is nocturnal, while the peak of larval feeding is diurnal. Our findings closely correlate with Water’s data on the expression of the circadian gene Per and feeding behavior. Waters et al. found that Per expression was higher in the early morning (ZT1) and lower in the late afternoon (ZT13) [13]. Similarly, our data showed that Tim expression was higher in the early morning (ZT3) and lower in the late afternoon (ZT15). This close correspondence between levels of Per and Tim at similar times of day indicates that PER and TIM proteins could be forming heterodimers in the cytoplasm of Manduca sexta, which constitutes a key part of the transcription-translation feedback loop.
In addition to our Tim gene expression data being consistent with the Per expression data reported in Waters et al., the expression pattern of Tim that we observed, being lower later in the day and higher in the morning, corresponds with the feeding patterns Waters et al observed in the early morning and in the late afternoon [13]. Hence, the synchrony of the Per and Tim rhythmicity may indicate a circadian role in feeding behavior during the day.
As previously mentioned, adult Manduca sexta has a functioning circadian clock that utilizes the same genes for influencing cycles of behavior [2], it is reasonable to predict that the same mechanism could influence its 5th instar feeding behavior. Previous research on other species, such as the Cotton Leafworm Spodoptera littoralis came to similar findings [6], with the researchers ultimately concluding that circadian rhythms were underpinning both the metabolism and feeding behavior of caterpillars of that species, indicating that there is a precedent for circadian rhythms being present in the larval stages of moths.
While the findings of this experiment were indicative of a functioning circadian clock, further studies would be required to cement our understanding of these mechanisms in the larvae. As circadian rhythms are endogenous and retain functionality in the absence of external cues, further testing in constant dark conditions (D:D) would be needed to determine whether the rhythmicity that we observed is truly circadian, meaning it is endogenous to the caterpillar, not dictated by light-dark cycles.
Acknowledgements
We would like to thank Dr. Hannah Pie who provided her time and aid in navigating the R program which yielded the statistical analysis results displayed in this paper. We would like to also thank Donovan Waters for harvesting the samples that we analyzed in this study. Our gratitude goes to all the professors and previous cohorts that made this project possible, thank you.
Contacts: rowan.cain@howardcc.edu, subeen.lee@howardcc.edu, matthew.quintanilla@howardcc.edu, kylie.rankin@howardcc.edu, emccarthy@howardcc.edu
References
[1] Bell-Pedersen D., Cassone V.M., Earnest D.J., Golden S.S., Hardin P.E., Thomas T.L., Zoran M.J. (2005). Circadian rhythms from multiple oscillators: lessons from diverse organisms. Nat Rev Genet., 6(7):544-56.
[2] Dunlap, J. C. (1999). Molecular Bases for Circadian Clocks, Cell, 96 (2), 271-290.
[3] Konopka R.J., Benzer S. (1971). Clock mutants of Drosophila melanogaster, Proc Natl Acad Sci U S A., 68(9):2112-6.
[4] Golombek, D. A., Rosenstein, R. E. (2010). Physiology of Circadian Entrainment, Physiological Reviews, 90(3), 1063-1102.
[5] Kolodny-Hirsch, D.M., Harrison, F. P. (1986). Yield Loss Relationships of Tobacco and Tomato Hornworms (Lepidoptera: Sphingidae) at Several Growth Stages of Maryland, Tobacco. J Econ Entomol, 79(3), 731–735.
[6] Zhang, J., Li, S., Li, W., Chen, Z., Guo, H., Liu, J., Xu, Y., Xiao, Y., Zhang, L., Arunkumar, K. P., Smagghe, G., Xia, Q., Goldsmith, M. R., Takeda, M., & Mita, K. (2021). Circadian regulation of night feeding and daytime detoxification in a formidable Asian Pest Spodoptera litura. Communications Biology, 4(1), 286.
[7] Tomioka K, Matsumoto A. (2010).A comparative view of insect circadian clock systems. Cell Mol Life Sci., 67(9):1397-406.
[8] Niepoth N, Ke G, de Roode JC, Groot AT. (2018). Comparing Behavior and Clock Gene Expression between Caterpillars, Butterflies, and Moths. J Biol Rhythms, 33(1):52-64.
[9] Bloch, G., Barnes, B. M., Gerkema, M. P., Helm, B. (2013). Animal activity around the clock with no overt circadian rhythms: patterns, mechanisms and adaptive value. Proceedings. Biological sciences, 280(1765), 20130019.
[10] Benito, J., Zheng, H., Ng, F.S., Hardin, P. E. (2007) Transcriptional feedback loop regulation, function and ontogeny in Drosophila, Cold Spring Harbor Symposia on Quantitative Biology, 72. 437-44, 2007.
[11] Fenske, M.P., Nguyen, L.P., Horn, E.K. Riffell, J.A., Imaizumi, T. (2018) Circadian clocks of both plants and pollinators influence flower seeking behavior of the pollinator hawkmoth Manduca sexta. Sci Rep, 8, 2842.
[12] Schuckel, J., Siwicki, K.K. Stengl, M. (2007). Putative circadian pacemaker cells in the antenna of the hawkmoth Manduca sexta, Cell Tissue Res, 271–278.
[13] Waters D. (2022). Manduca sexta Larvae Exhibit Rhythms in Feeding Behavior and Rhythms in Gene Expression of the Circadian Gene Period, Journal of Research in Progress, 5, 2-12.
[14] Green, M., Jun, J., McCarthy, E., Pie, H. (2021). Molecular Mechanisms for Circadian Rhythms in the Tobacco Hornworm (Manduca sexta). Journal of Research in Progress, 4, 24–34.
[15] Arhampong, A., Arminio, M., Bucksell, J., Deljookorani, S., Tegeredegn, B. (2019). Neuropeptide F mRNA Expression in the Tobacco Hornworm. Journal of Research in Progress, 2, 12-19.
[16] Walla, T. R., Greeney, H. F. (2012). Under cover of darkness, caterpillars take flight: the immature stages and feeding ecology of the glasswinged butterfly, Oleria baizana in eastern Ecuador. Journal of Insect Science (Online), 12, 106.