|
Is Saccharomyces cerevisiae a Viable Model for Studying Cancer Mutations in KRAS? Arsany Amgad Doud, Howard Community College |
Abstract
Saccharomyces cerevisiae has been used as a model organism for many diseases as approximately 1/3 of the Saccharomyces cerevisiae genome has human homologs and the related proteins also have about a 32% amino acid similarity to humans[4]. The question if Saccharomyces cerevisiae can be a model for cancer is discussed here. Mutations in KRAS are implicated in many human cancers and was one of the first isolated human oncogenes. Saccharomyces cerevisiae has a homolog to KRAS, named RAS1, and the sequence is highly conserved. Bioinformatics methods were used to predict primary, secondary, and tertiary structures of the mutant protein to see if the RAS1 mutant would be a viable model to mimic the mutations of KRAS. Here it is shown that the predicted structures of the mutant are comparable to the structure of RAS1 and indicate that RAS1 would be a viable model to mimic KRAS justifying the use of Saccharomyces cerevisiae as a model for cancer cells.
Introduction
Cancer research has existed for centuries, and, in the past 100 years, there have been many advances, but there still is a lot about cancers that are poorly understood. One such advancement was the discovery of proto-oncogenes. A proto-oncogene is a gene involved in normal cell growth. Mutations in proto-oncogenes can induce uncontrollable growth leading to cancer. The KRAS gene was one of the first isolated human oncogenes, and its mutations are present in 25% of all tumors including in 32% of lung cancers, 40% of colorectal cancers, and 90% of pancreatic cancers[6]. The KRAS protein is imbedded in the cell membrane and wild type KRAS proteins cycle between on and off by binding to guanosine triphosphate (GTP) and converting it to guanosine diphosphate (GDP). KRAS activity is an early step in the cascade leading to other signaling proteins to initiate certain gene expressions to initiate the cell cycle progression, cell proliferation, RNA and protein transport, and actin organization[7]. Certain mutations lead the KRAS protein to always be active (constantly bound to GTP). The overactive KRAS protein leads uncontrolled gene cell cycle progression and cell proliferation which can lead to cancer. KRAS has been considered “untargetable” because of its small size, smooth surface, and the constantly bound GTP[8].
The most common mutation in KRAS is in codon 12 which codes for glycine (G12). The majority of the mutations in the amino acid are glycine to cysteine (G12C; 39% in lung cancers), glycine to valine (G12V), or glycine to aspartic acid (G12D) and a small percentage of other mutations[9]. Glycine-12 is located next to the active site where KRAS binds GTP but is not part of the active site[9]. Since it is the most common mutation and the significant differences in the side chains, we chose to look at the G12C mutation. Glycine is a nonpolar amino acid, while cystine is a polar, neutral amino acid with a thiol group (sulfur-hydrogen) as its side chain and a much larger molecular weight (MW) (Figure 1).

Although for many years, KRAS has mostly been considered “untargetable” because its unusual shape made it difficult to design drugs to bind to it. Over the past several years, drugs that bind to and inhibit the KRAS with the G12C mutation have been developed. Several of these are in clinical trials, and one of these, Sotorasib, was approved in December 2022. Sotorasib is highly effective in treating patients with advanced non-small-cell lung cancer (NSCLC) who have a specific KRAS mutation known as KRAS G12C[10]. The study found that Sotorasib binds covalently to KRAS G12C. Sotorasib works by binding to a specific pocket on the KRAS protein and preventing it from becoming active by inducing a conformational change in KRAS. Unfortunately, these drugs only work with KRAS with the G12C mutation; it does not work on KRAS with other mutations. In addition, although this drug works well initially, over time the cancer cells become resistant to the drug.
Proteins are characterized by primary structure (amino acid chain), secondary structure (folding based on hydrogen bonding of the backbone) and can form β-strands, α-helices, and loops, and a tertiary structure (three-dimensional folding based on the interactions between the side chains). By analyzing the KRAS protein and comparing it to the Saccharomyces cerevisiae homolog, RAS1, it would be possible to determine if RAS1 would be a viable model for studying KRAS. Importantly, once RAS1 is mutated, it could lead to different secondary and tertiary structures as an amino acid on the primary structure changes.
Saccharomyces cerevisiae (baker’s yeast) has been used as a model organism for many diseases as approximately 1/3 of the Saccharomyces cerevisiae genome has human analogs and the related proteins also have about a 32% amino acid similarity to humans[4]. Studies include aging, cell cycle control, DNA damage response, metabolism, and signal transduction[4, 11]. Saccharomyces cerevisiae has a KRAS homolog called RAS1. The sequences of RAS1 and KRAS are highly conserved, especially in the active site. It is important to realize that G12 in KRAS will not necessarily be G12 in RAS1. In this paper, bioinformatics approaches were used to predict the conformation of RAS1 carrying a G to C mutation would be the same or similar to KRAS. The alignment of the genes and amino acids, predictions of secondary and tertiary structures were then compared to KRAS to determine if RAS1 would be a viable model to proceed with wet lab testing.
Methods
Multisequence alignment. Using Clustal Omega[5], a sequence alignment was done to determine the similarities between human KRAS protein (NP_001356715.1) and Saccharomyces Cerevisiae RAS1 protein (NP_014744.1). The protein sequences were obtained courtesy of the National Library of Medicine.
Comparison of Secondary Structures. Since the crystal structure of RAS1 is known, the secondary structure is also available. By changing the sequence of G12C in RAS1, we were able to predict the secondary structure using PsiPred prediction database[12] and compared it to the known secondary structure of RAS1.
Comparison of Tertiary Structures. Since the secondary structures of RAS1 and the mutated sequence (G12C) were slightly different, we used AlphaFold Protein Structure Database to predict the tertiary structure of our mutant protein and compared it to the known tertiary structure of RAS1[13].
Results
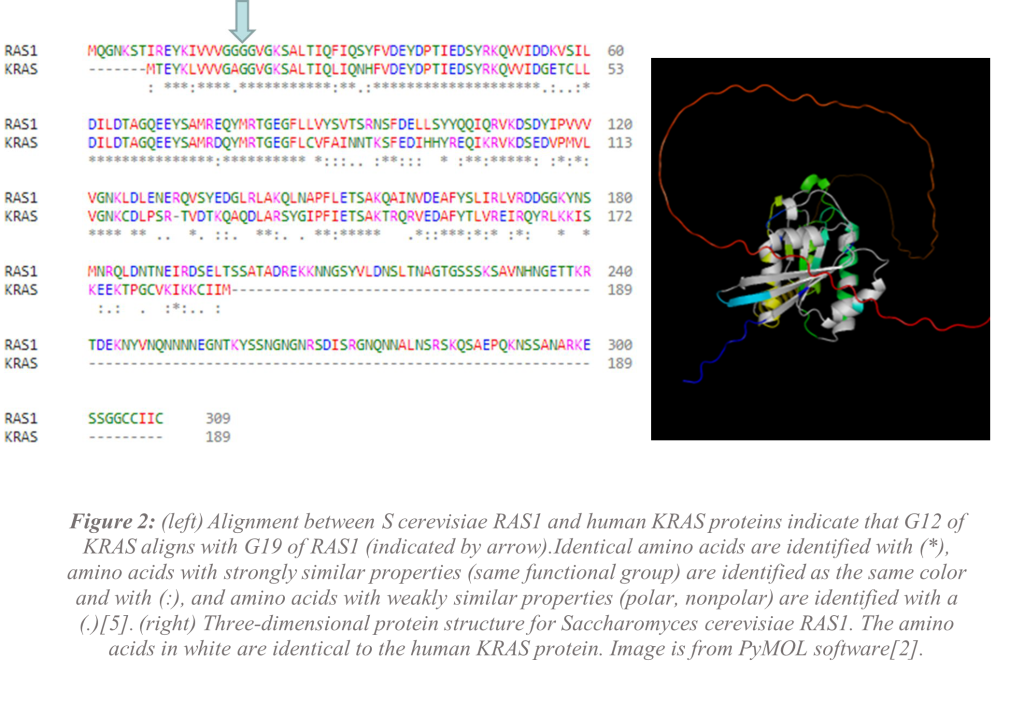
The alignment of the human KRAS protein and Saccharomyces Cerevisiae RAS1 protein shows that glycine-12 in human KRAS aligns with glycine-19 in RAS1 (Figure 2). RAS1 is much larger than KRAS, but the proteins are highly conserved (similar) at the N-terminal end (beginning) of the proteins. While RAS1 has more than 100 additional amino acids than KRAS, 80% of the amino acids are similar including about 60% of the amino acids being identical[14]. Glycine-19 appears to be a good target for mutation but knowing how the protein folds in its secondary and tertiary structures will be necessary to understand if RAS1 would be a good model for KRAS. Comparison of the secondary structures using the known structure for wild-type (WT) RAS1 and the selected mutant RAS1 G19C shows that there are no differences in the secondary structure of the active sites of the proteins (Figure 3) indicating that the mutant would likely still function. This mutant could explain why mutant KRAS is always “on.” There are slight differences in the amino acids involved in β-strands and α-helices as well as varied sizes in the structures.

The three-dimensional folding of the WT RAS1 compared to RAS1 G19C mutant shows that the active sites on both proteins are similar to each other in shape and provides more evidence that the protein would be still able to function. However, there are differences in the folding of the protein (Figure 4). The differences in folding could possibly be the reason GTP is permanently bound to the mutant KRAS protein and would be supported if GTP is permanently bound to the RAS1 G19C protein. The entire protein outside of the active site is known as the allosteric site. Changes in the allosteric site could possibly mean a tighter bind to the GTP preventing the protein from properly functioning leading to the uncontrolled activation.

Discussion and Conclusion
The comparison that was shown in (Figure 2) indicates the human KRAS G12 overlaps with Saccharomyces cerevisiae RAS1 at G19. The predicted models of the RAS1 G19C mutant show that there are similarities in WT RAS1 and RAS1 G19C mutant. The differences in the secondary structures (Figure 3) and tertiary structures (Figure 4) do not affect the active site but could indicate how a mutation in RAS1 could leave the protein turned “on” and always bound to GTP. Since RAS1 G19C mutation is not at the active site but is the amino acid immediately preceding the active site, we believe that the preliminary data supports the hypothesis that Saccharomyces cerevisiae would be a viable model for studying cancer mutations in KRAS.
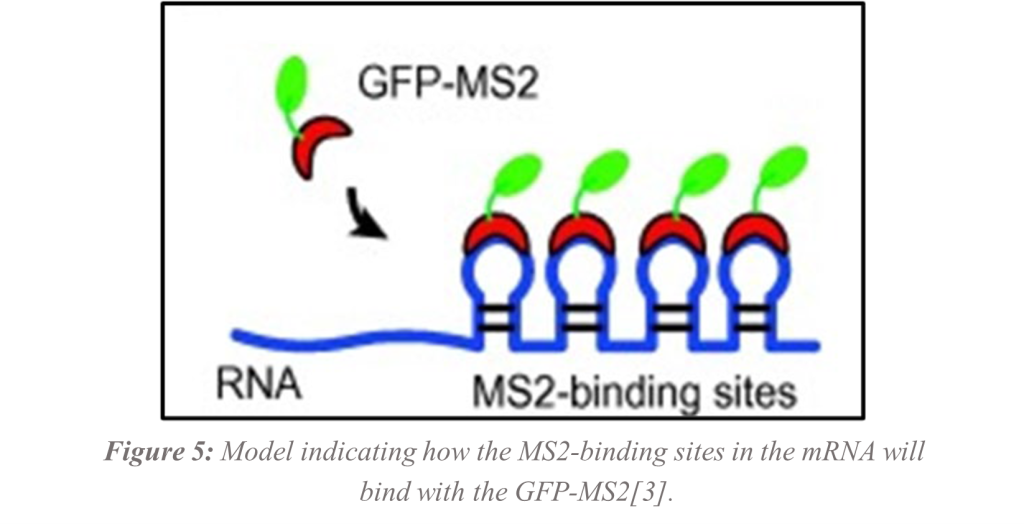
Now that it appears that RAS1 will be a good model for human KRAS, the next steps will be to insert the RAS1 gene into expression vectors (plasmids) and perform site-directed mutagenesis in G19. Future studies will have the plasmids inserted into Saccharomyces Cerevisiae and visualized under the fluorescent microscope. The mRNA for the RAS1 protein using single molecule fluorescent in situ hybridization (smFISH) will be visualized using the MS2 bacteriophage protein binding sites containing the RAS1 gene in one plasmid tagged with green fluorescent protein (GFP) bound to the MS2 bacteriophage protein from the second plasmid. The binding of the GFP-MS2 to the target mRNA with the MS2-binding sites will allow visualization of individual RNA molecules[3, 15] (Figure 5). The long-term goal would be to use site directed mutagenesis to mutate the cDNA (copy DNA from mRNA). The hypothesis is that this would lead to uncontrollable proliferation. Once successful, smFISH will be used in live cells with the mutated RAS1 again visualizing individual mRNA molecules. Finally, small molecules will be used to target the mRNA with the goal of preventing translation. By targeting the mRNA, many problems associated with targeting the KRAS protein may be eliminated.
Contact: arsany.doud@howardcc.edu, arinze.ezeifeka@howardcc.edu, jsparenberg@howardcc.edu
References
[1] T. U. Consortium et al., “UniProt: the universal protein knowledgebase in 2021,” Nucleic Acids Research, vol. 49, no. D1, 2020, doi: 10.1093/nar/gkaa1100.
[2] The PyMOL Molecular Graphics System, Version 2.0 Schrödinger, LLC. (2022). [Online]. Available: https://pymol.org/2/support.html?
[3] Z. Ma, X. Wu, C. J. Krueger, and A. K. Chen, “Engineering Novel Molecular Beacon Constructs to Study Intracellular RNA Dynamics and Localization,,” Genomics, Proteomics & Bioinformatics, vol. 15, no. 5, pp. 279-286, 2017, doi: https://doi.org/10.1016/j.gpb.2017.04.004.
[4] L. Vanderwaeren, R. Dok, K. Voordeckers, S. Nuyts, and K. J. Verstrepen, “Saccharomyces cerevisiae as a Model System for Eukaryotic Cell Biology, from Cell Cycle Control to DNA Damage Response,” (in en), International Journal of Molecular Sciences, Review vol. 23, no. 19, p. 11665, 2022-10-01 2022, doi: 10.3390/ijms231911665.
[5] S. F et al., “Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega,” Molecular systems biology, vol. 7, 10/11/2011 2011, doi: 10.1038/msb.2011.75.
[6] L. P, W. Y, and L. X, “Targeting the untargetable KRAS in cancer therapy,” Acta pharmaceutica Sinica. B, vol. 9, no. 5, 2019 Sep 2019, doi: 10.1016/j.apsb.2019.03.002.
[7] L. H. Kim HJ, Jeong MS, Jang SB., “Oncogenic KRAS: Signaling and Drug Resistance,” Cancers (Basel), vol. 13, no. 22, 2021, doi: https://doi.org/10.3390%2Fcancers13225599.
[8] W. A, A. A, and R. AU, “Strategies for Targeting KRAS: A Challenging Drug Target,” Current pharmaceutical design, vol. 28, no. 23, 2022 2022, doi: 10.2174/1381612828666220506144046.
[9] H. A. Yu et al., “Prognostic Impact of KRAS Mutation Subtypes in 677 Patients with Metastatic Lung Adenocarcinomas,” (in English), Journal of Thoracic Oncology, vol. 10, no. 3, pp. 431-437, 2015/03/01 2015, doi: 10.1097/JTO.0000000000000432.
[10] L. B. Skoulidis F, Dy GK, Price TJ, Falchook GS, Wolf J, Italiano A, Schuler M, Borghaei H, Barlesi F, Kato T, Curioni-Fontecedro A, Sacher A, Spira A, Ramalingam SS, Takahashi T, Besse B, Anderson A, Ang A, Tran Q, Mather O, Henary H, Ngarmchamnanrith G, Friberg G, Velcheti V, Govindan R., “Sotorasib for Lung Cancers with KRAS p.G12C Mutation,” N Engl J Med, vol. 384, no. 25, pp. 2371-2381, 2021, doi: https://doi.org/10.1056%2FNEJMoa2103695.
[11] V. E. Karathia H, Sorribas A, Alves R., “Saccharomyces cerevisiae as a model organism: a comparative study.,” PLoS One., vol. 6, no. 2, 2011, doi: https://doi.org/10.1371%2Fjournal.pone.0016015.
[12] J. DT, “Protein secondary structure prediction based on position-specific scoring matrices,” Journal of molecular biology, vol. 292, no. 2, 09/17/1999 1999, doi: 10.1006/jmbi.1999.3091.
[13] J. Jumper et al., “Highly accurate protein structure prediction with AlphaFold,” (in En), Nature, OriginalPaper vol. 596, no. 7873, pp. 583-589, 2021-07-15 2021, doi: doi:10.1038/s41586-021-03819-2.
[14] P. F. Cazzanelli G, Alves S, Francisco R, Azevedo L, Dias Carvalho P, Almeida A, Côrte-Real M, Oliveira MJ, Lucas C, Sousa MJ, Preto A., “The Yeast Saccharomyces cerevisiae as a Model for Understanding RAS Proteins and Their Role in Human Tumorigenesis,” Cells, vol. 7, no. 2, 2018, doi: https://doi.org/10.3390%2Fcells7020014.
[15] E. Tutucci, M. Vera, J. Biswas, J. Garcia, R. Parker, and R. Singer, “An improved MS2 system for accurate reporting of the mRNA life cycle.,” Nat Methods, vol. 15, no. 1, pp. 81-89, 2018, doi: 10.1038/nmeth.4502.