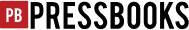6 Species-Specific and Generalized Inductions of Preference in Manduca sexta
Mary N. Lenahan, Howard Community College
Pilar D. Thomas, Howard Community College
Mentored by: William C. Gretes, Ph.D.
Abstract
Diet-induced changes in the feeding preferences of Manduca sexta caterpillars have been observed in a number of studies. In nature, these caterpillars feed almost exclusively from plants in the family Solanaceae, however, larvae can also be successfully raised in a laboratory setting on select non-solanaceous foods. In the current study, two types of feeding inductions (“species-specific” and “generalized”) were explored using primarily non-solanaceous plants.
Larvae were reared in a greenhouse on rapeseed (Brassica napus), turnip (Brassica rapa), and cabbage (Brassica oleracea) from the family Brassicaceae; cowpea (Vigna unguiculata) and pea (Pisum sativum) from the family Fabaceae; and tobacco (Nicotiana tabacum) from the family Solanaceae. Daily records of larval mortality were recorded to determine plant-dependent larval survivability. Freshly-molted fifth instar larvae were recorded using cameras during a no-choice test in which they were given a single leaf from a test plant. Percent consumption was visually analyzed over a 10-hour period. Our feeding assay data provide evidence for: (1) species-specific inductions of preference for cowpea and turnip, as well as (2) generalized induction of preference for pea-reared larvae on cowpea, cowpea-reared larvae on pea, and rapeseed-reared larvae on turnip.
We conclude that both species-specific and generalized inductions of preference exist in some, but not all, non-solanaceous plant rearing and testing combinations. An analysis of larval survivorship indicates that some plants were far more suitable as host plants for M. sexta larvae than others. Larvae reared on rapeseed, turnip, cowpea, or tobacco were more likely to survive to the fifth instar, whereas larvae reared on pea or cabbage experienced significantly higher mortality. Our findings expand the understanding of inductions of preference and reveal that the mechanism for these inductions may be more complex than concluded by previous studies.
Introduction
The larval stage of the Manduca sexta moth, also known as the tobacco hornworm, has been the subject of many feeding behavior studies. The Manduca sexta larvae progresses through five molting stages (instars) where they grow and develop. In nature, these caterpillars feed almost exclusively from plants in the family Solanaceae, leading to the caterpillars being classified as oligophagous herbivores. Despite that, M. sexta larvae can be successfully raised in a laboratory setting on Solanaceous plants as well as a few non-solanaceous foods.
Many studies have been performed to try to determine if the tobacco hornworm’s apparent preference for Solanaceous plants is innate or induced; yet, after decades of research the answer is still unclear. Some studies suggest that the nature of the larvae’s oligophagy must be induced [1], while others suggest it is innate [2,3]. However, there are no specified conditions that must be met for an organism to be considered oligophagous, so how the term is used in scientific literature is at the discretion of the author, as pointed out by Gretes et al. [3]. Yamamoto [1] concluded that larvae were oligophagous only if they fed exclusively on a Solanaceous plant in the presence of non-solanaceous alternatives. Other researchers’ use of the term oligophagy were not as rigid; de Boer & Hanson [2] concluded that caterpillars were oligophagous if they showed a strong preference for Solanaceous plants during choice tests [1,2]. Similarly, another study concluded that M. sexta larvae were oligophagous since they showed high acceptability for Solanaceous plants during no-choice tests, regardless of their rearing experience [3]. It is generally believed that newly hatched larvae in the 1st instar that lack any prior feeding experience prefer Solanaceous plants over non-solanaceous foods. In one study, 60-75% of 1st instar larvae chose the Solanaceous plant tomato over the non-solanaceous alternatives (mullein, collard, and cowpea) [1]. Another test found that around 70-80% of 1st instar larvae chose tomato over the non-solanaceous plants: cowpea, radish, and rapeseed [2]. Yamamoto and de Boer & Hanson had similar findings but, since they defined oligophagy in a different way, came to opposing conclusions regarding innate and induced feeding behaviors since they did not define oligophagy in the same way [1,2].
While these studies differ in their definition of oligophagy, all reached the same conclusion that the caterpillar’s feeding preferences can be modified by diet experience. Studies have shown that rearing the hornworms on a plant species makes that plant species more palatable to the larvae [1,2,3,4], termed an “induction of specific food preference” by Jermy et al., [4]. For example, this phenomenon has been repeatedly demonstrated with cowpea, a non-solanaceous plant that 1st instar larvae do not find particularly palatable [1,2,3]. de Boer and Hanson [2] found that when larvae were reared on cowpea to the 5th instar, those larvae ate significantly more cowpea, even in the presence of a Solanaceous alternative. Other experiments have yielded data that supports these findings [1,3,6].
Inductions of preference for both Solanaceous and non-solanaceous plants have been observed [2,3]. de Boer & Hanson [2] concluded that the strength of the induction of preference between two plants was affected by the relatedness of those plants. One study found that rearing larvae on cowpea, in addition to inducing a preference for cowpea, also increased the acceptability for another closely related plant in the same genus, corkscrew vine [3]. Corkscrew vine is highly rejected by M. sexta, yet cowpea-reared larvae readily consumed it. This was the first recorded instance of an increase in the acceptability of a non-solanaceous plant by rearing larvae on a similar, but related, non-solanaceous plant.
Two of the studies mentioned above used multi-choice tests to determine feeding preferences [1,2]. Such multi-choice tests prioritize showing preference over acceptability and cannot indicate the level of acceptability for each test food. Individual plant acceptability can be shown by using a no-choice test which compares the time before feeding as initiated, the amount eaten, and the rate of consumption [3].
In order to examine an induction of acceptability between non-solanaceous related plants, we tested for induction of related preference for rapeseed, turnip, and cabbage from the family Brassicaceae, in addition to cowpea and pea from the family Fabaceae. We hypothesize that rearing larvae on one non-host plant will increase its induction of acceptability for another related non-host plant. For example, if our hypothesis is correct, we would expect rapeseed-reared larvae to show an increase of acceptance for turnip and cabbage. Likewise, cowpea-reared larvae will show an increase of acceptance for pea and vice versa. If our hypothesis is incorrect, we would not expect to see an increase of acceptance in the situations mentioned above. In this study we explored inductions of preference and attempted to distinguish the changes in feeding behavior by categorizing them using two new terms:
- “Species-specific”, in which larvae show an increased acceptance of a particular species of a plant when previously reared on that plant.
- “Generalized”, in which larvae show an increased acceptance of a particular species when reared on a different, closely related species in the same family.
This research is significant because the results of induction of related preference in non-host plants for M. sexta is largely unknown as it has scarcely been explored. The principal articles demonstrate induction of preference for acceptable host plants. The data will contribute to this field of study and improve biologist’s understanding of Manduca sexta.
Materials and Methods
Materials used for the experimentations were as follows; plant pots, soil, seed packets (rapeseed (Brassica napus variant Dwarf Essex Rape), turnip (Brassica rapa subsp. rapa; variant Purple-Top White Globe), and cabbage (Brassica oleracea; variant Early Jersey Wakefield) from the family Brassicaceae; cowpea (Vigna unguiculata; variant California Blackeye No. 5) and pea (Pisum sativum; variant Blue Bantam) from the family Fabaceae; and tobacco (Nicotiana tabacum), Manduca sexta larvae eggs, and rearing containers. The experiment arena platform materials were as follows; handmade black metal platform with six short plastic hollow prongs, small white circular plastic disk, small white plastic grid, a large clear plastic petri dish, small metal pin, and high-definition cameras (Figure 1).
Rearing methods included daily check-ins on each plant type. Each plant cohort was cultivated in the greenhouse where the temperature, light, and air quality were controlled in order to create the most consistent and viable environment for all plants. The greenhouse maintained sufficient temperature and humidity and water provided to plants on a daily basis. All plants were also monitored for health and viability on a daily basis. The larval eggs were provided by Carolina Biological Supply [5]. Rearing methods for each larval cohort followed a similar procedure of daily check-ins and assessment of health in order to assess viability and growth, specifically molting. Five newly hatched larvae were put in each plastic container specified by the plant cohort. The plastic containers were cleaned daily by rinsing and hand drying to remove any debris (fecal matter, dead caterpillars, and plant residue). Within the plastic container would not only be the larvae, but the plant foliage, which was also monitored and replaced daily. Plant foliage was obtained during the daily plant check-in where a healthy, viable, single piece would be cut from the stem of a specific plant. A small piece of a moistened paper towel would be wrapped around the stem of the foliage to prevent withering. The plants from which foliage were cut were discarded as cutting them could change the chemical composition of the plant and therefore alter the results of the study [6].
When the Manduca sexta larvae were in the premolt stage between the fourth and fifth instars, they were transferred into a separate plastic container for 24 hours to allow them to finish molting into the fifth instar in preparation to be tested in the experimental arena. The feeding behavior tests were performed on a custom setup. The base of the setup was a handmade black metal platform with six short plastic hollow prongs in a circular shape. The platform could hold three separate circular arenas at once. Each arena consisted of a circular disk of thin white plastic, (to add contrast for better viewing with the cameras), a circular disk of white plastic grid (to make it easier for the larvae to walk around), and a clear plastic petri dish (to act as a lid over the arena). A fresh leaf from one type of plant (from the family Brassicaceae, Fabaceae, or Solanaceae) was placed in the testing area with the larvae. The leaf was secured in place using a metal pin, inserted through the stem of the leaf into one of the hollow platform prongs. The larval rearing cohort and test plant species were recorded as the rearing and test plant combination for each larva. High-definition cameras captured raw images every 30 seconds over a 24-hour period.
After each 24-hour experiment was completed, the raw images were transferred onto a desktop computer where the images were examined by eye to determine the time at which the caterpillar first moved. The image that showed the larva’s first movement was recorded as image #1. The image taken 60 minutes after image #1 was deemed image #2, and this process was repeated until there were 10 images taken over a 10-hour period. These 10 images were visually analyzed by eye and the plant consumption percentage that was estimated by eye was recorded for each photo. Consumption data for each experiment was electronically recorded in Microsoft Excel. The data from each experiment was categorized according to the specific rearing and test plant combination. The hourly consumption data for each trial of a rearing and test plant combination was averaged and plotted on graphs using Microsoft Excel.

Results
Feeding Behavior
Larvae reared on different types of plants were tested on cabbage. Cabbage-reared and rapeseed-reared larvae consumed cabbage the most, whereas the tobacco-reared and turnip-reared larvae consumed cabbage the least, and cowpea-reared larvae consumed cabbage moderately. Pea-reared larvae were not tested due to the lack of pea-reared and cabbage testing combination (Figure 2b).
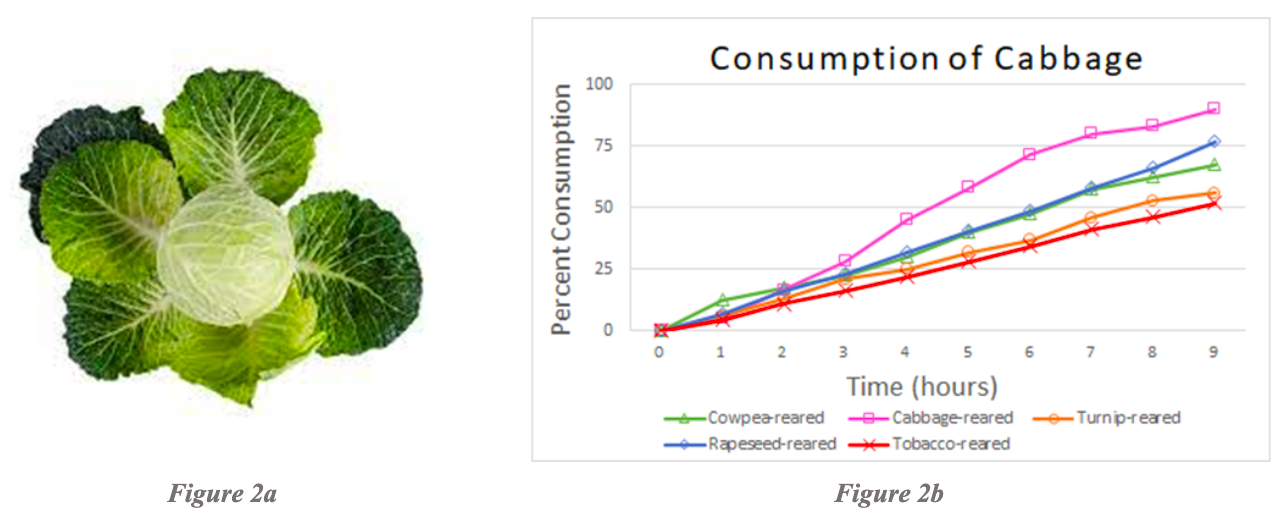
Examination of consumption of turnip leaves revealed that rapeseed-reared larvae consumed turnip the most over a 10-hour period of time, and the turnip-reared larvae consumed turnip almost as much as rapeseed-reared larvae (Figure 3b). Tobacco-reared larvae consumed turnip the least over a 10-hour period. Cabbage-reared, pea-reared, and cowpea-reared larvae were not tested due to the lack of testing and rearing combinations for turnip.
( n = 13).
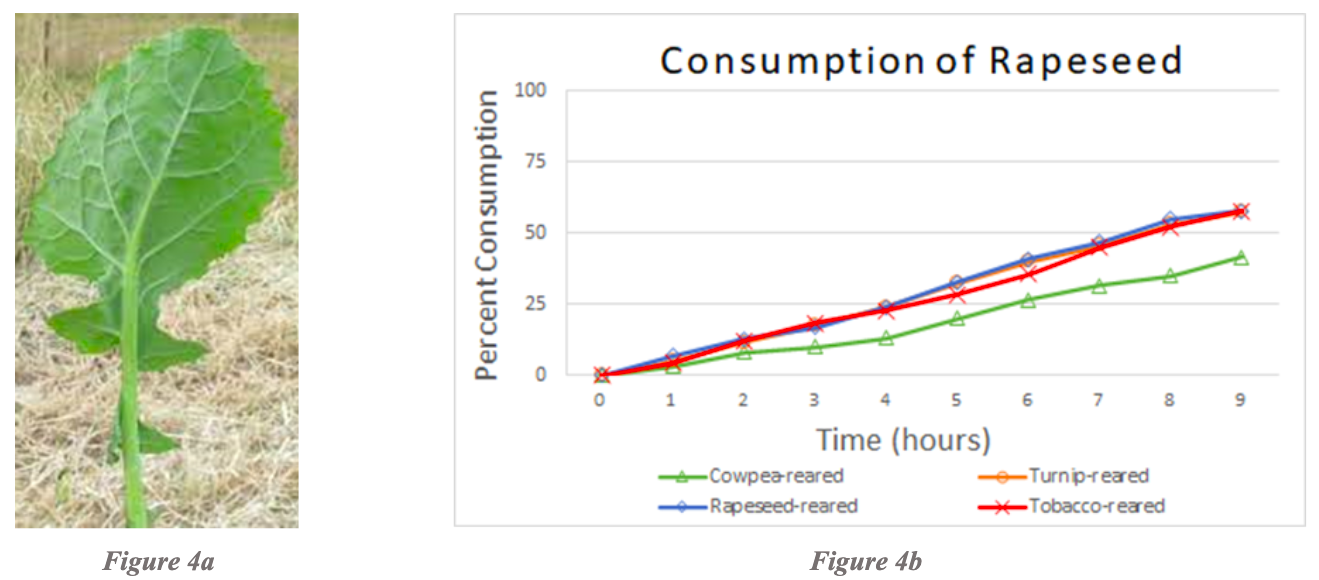
Studies of consumption of rapeseed revealed that rapeseed-reared, turnip-reared, and tobacco-reared larvae consumed rapeseed at the same rate (Figure 4b). Cowpea-reared larvae consumed less rapeseed than the rest of the rearing cohorts, but still consumed a modest amount. Pea-reared and cabbage-reared were not tested due to the lack of testing and rearing combinations for rapeseed.
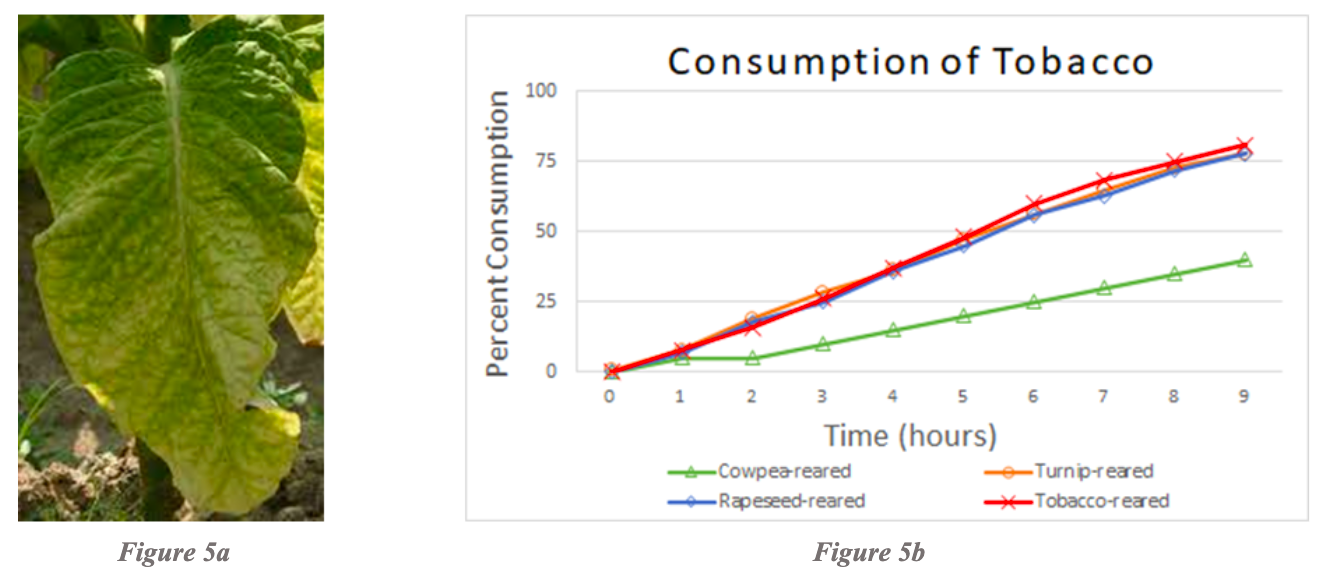
Examination of tobacco consumption revealed that tobacco-reared, turnip-reared, and rapeseed-reared larvae consumed high amounts of tobacco at the same rate. Cowpea-reared larvae consumed significantly less tobacco, yet still ate a moderate amount of tobacco (Figure 5b). Pea-reared and cabbage-reared larvae were not tested due to the lack of testing and rearing combinations for tobacco.
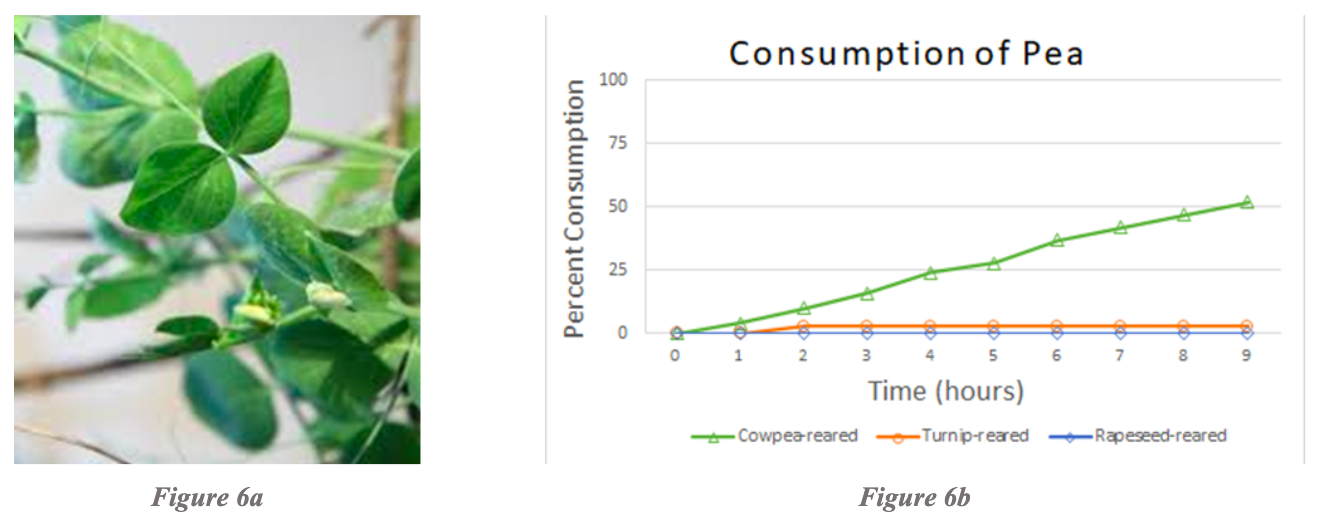
Examination of pea consumption revealed that cowpea-reared larvae consumed pea the most over a 10-hour period of time whereas rapeseed-reared and turnip-reared larvae consumed little to no pea at all (Figure 6b). Note that it is also evident within cowpea-reared larvae on pea that cowpea-reared larvae were the only cohort to consume pea among those tested. Cabbage-reared, pea-reared, and tobacco-reared larvae were not tested due to the lack of rearing and testing combinations for pea.
(n = 5).
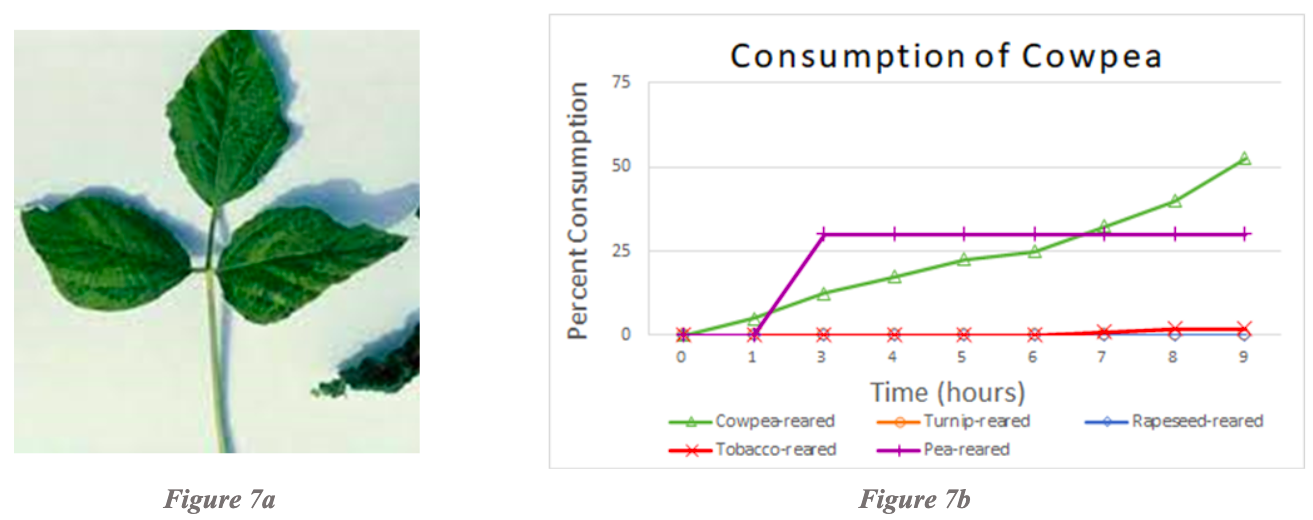
Examination of cowpea consumption revealed that pea-reared and cowpea-reared larvae consumed cowpea the most over a 10-hour period of time, whereas turnip-reared, rapeseed-reared, and tobacco-reared larvae consumed almost no cowpea (Figure 7b). Testing pea-reared larvae on cowpea revealed that pea-reared larvae were the only cohort to consume cowpea. Cabbage-reared larvae were not tested due to the lack of testing and rearing combinations for cowpea.
Larval Survivorship
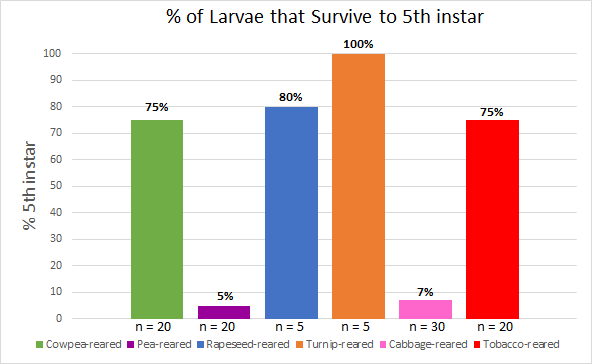
In order to determine which plants were better suited to support larval life to the fifth instar, larval mortality was recorded for each rearing cohort during the daily larval care regimen. The data was graphed using Microsoft Excel as the percentage of larvae from each rearing cohort that survived to the fifth instar (Figure 8). Larvae from the cowpea-reared, rapeseed-reared, turnip-reared, and tobacco-reared cohorts had a high survival rate of at least 75%, whereas only 5% of pea-reared larvae, and 7% of cabbage-reared larvae survived to the fifth instar.
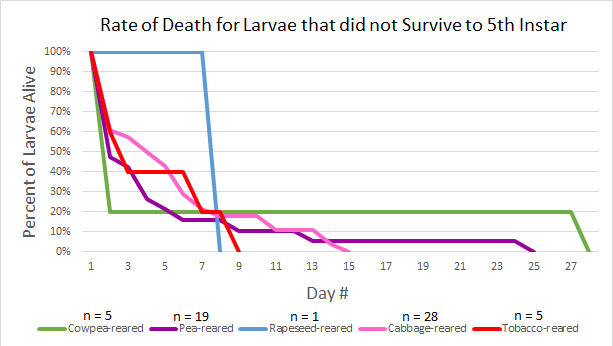
The survivorship of larvae that did not survive to the fifth instar from each rearing cohort was graphed in order to compare the timing of mortality across rearings (Figure 9). Turnip-reared larvae are not represented in the rate of death graph since 100% of the larvae from that cohort survived to the fifth instar.
Discussion
In this study, species-specific and generalized inductions of preference were observed in some, but not all, non-solanaceous plant rearing and testing combinations. Most notably, species-specific inductions of preference were evident for cowpea (Figure 7b). Generalized inductions of preference were evident when testing pea-reared larvae on cowpea (Figure 7b), as well as cowpea-reared larvae on pea (Figure 6b). As both of these plants belong to the same plant family (Fabaceae), this is a clear example where rearing on one plant increases the palatability of other, closely related plants. Generalized induction of preference was also evident when testing rapeseed-reared larvae on turnip (Figure 3b). Both rapeseed and turnip are part of the family Brassicaceae.
One of the challenges that affected this study was growing and maintaining enough healthy plants to support larval rearing and testing. Plants that showed signs of yellowing or browning of leaves, as well as excessive wilting, were discarded because the chemical composition of unhealthy plants differs from healthy ones, and therefore could affect the outcomes of larval rearing and testing. For example, many of the turnip plants grown in the greenhouse were not suitable, which is why the n-value for turnip-reared larvae was only 5.
Another challenge that affected this study was the low survivability rate of pea-reared and cabbage-reared larvae. This limited the number of larvae available from these cohorts to be tested and analyzed. As seen in Figure 8, only 5% of larvae reared on pea survived to the fifth instar, which severely limited the number of pea-reared larvae that were tested in the feeding behavior tests.
Due to difficulties with larval survivability we were unable to obtain the desired amount of feeding behavior data for pea-reared, cabbage-reared, and turnip-reared larvae. In addition, difficulties with plant vitality limited the use of turnip and pea as test plants; only 13 larvae were tested on turnip, and only 5 larvae were tested on pea (Compared to: Cabbage (n = 24), Rapeseed (n = 21), Tobacco (n = 24), and Cowpea (n = 16)). Future studies should seek to obtain at least 10 to 20 more tests for these plants based on the other counts for the other test plants.
Species-specific and generalized inductions of preference were observed in some, but not all, non-solanaceous plant rearing and testing combinations. Most notably, species-specific inductions of preference were evident for cowpea (Figure 7b). Generalized inductions of preference were evident when testing pea-reared larvae on cowpea (Figure 7b), as well as cowpea-reared larvae on pea (Figure 6b). As both of these plants belong to the same plant family (Fabaceae), this is a clear example where rearing on one plant increases the palatability of other, closely related plants. Generalized induction of preference was also evident when testing rapeseed-reared larvae on turnip (Figure 3b). Both rapeseed and turnip are part of the family Brassicaceae.
Conclusions
Our findings expand the understanding of inductions of preference and reveal that the mechanism for these inductions may be more complex than concluded by previous studies. These results are significant as they provide evidence for both generalized and species-specific inductions of preference. Our data support the conclusions of previous studies that stated that feeding inductions extend to other chemically similar plants, as some plants in families are more chemically similar than others [1]. However, our data does not support the conclusions of previous studies that stated that feeding inductions were the result of an increased sensitivity to a solanaceous-exclusive chemical [1,6].
As shown in Figure 8, these studies indicate that certain plants are significantly more successful in supporting larval life to the fifth instar than others. These results support conclusions made in previous studies [1,2].
Due to challenges of our study, we were unable to rear a desirable number of larvae on pea, which restricted our ability to collect feeding behavior data. Similarly, we were unable to test generalized inductions of preference for cabbage-reared larvae on turnip and rapeseed due to the low survival rate of cabbage-reared larvae. One focus of future studies will be on rearing desirable amounts of pea-reared and cabbage-reared larvae in order to generate more feeding behavior test data. It would also be advantageous to include soybean, a plant from the family Fabaceae, since only two plants from the family Fabaceae were used in our study.
Acknowledgements
We would like to thank the National Science Foundation for funding this research, the Undergraduate Research program leaders and the advisory board for providing us with this opportunity, Dr. William Gretes for his knowledge and leadership, and Caitlin Beckjord for her invaluable assistance maintaining the HCC greenhouse.
Contacts: mary.lenahan@howardcc.edu, pilar.thomas@howardcc.edu, wgretes@howardcc.edu
References
[1] R. T. Yamamoto, “Induction of Hostplant Specificity in the Tobacco Hornworm, Manduca sexta,” Journal of Insect Physiology, vol. 20, no. 4, pp. 641-650, 1974.
[2] G. de Boer and F. E. Hanson, “Food-plant selection and induction of feeding preference among host and non-host plants in larvae of the tobacco hornworm Manduca sexta,” Entomologia Experimentalis et Applicata, vol. 35, no. 2, pp. 177-193, 1984.
[3] W. C. Gretes, E. A. Stanwyck and F. E. Hanson, “Innate and acquired components of oligopoly in the herbivorous lepidopteran, Manduca sexta,” Entomologia Experimentalis et Applicata, vol. 160, no. 3, pp. 259-271, 2016.
[4] T. Jermy, F. E. Hanson and V. G. Dethier, “Induction of Specific Food Preference in Lepidopterous Larvae,” Entomologia Experimentalis et Applicata, vol. 11, no. 2, pp. 211-230, 1968.
[5] H. Carlson, A. Livingston, R. Mahmud, M. Huang, M. Fadudu, K. Samieniejad and W. C. Gretes, “Feeding Behaviors of Manduca sexta,” Journal of Research in Progress, vol. 3, pp. 45-55, 2020.
[6] M. L. del Campo C. and A. A. Renwick, “Dependence on host constituents controlling food acceptance by Manduca sexta larvae,” Entomologia Experimentalis et Applicata, vol. 93, no. 2, pp. 209-215, 1999.
[7] D. V. Savatin, G. Gramegna, V. Modesti, and F. Cervone, “Wounding in the plant tissue: the defense of a dangerous passage,” Frontiers of Plant Science, vol. 5, article 470, pp. 1 – 11, 2014