Feeding Behaviors of Manduca sexta
Hartley Carlson, Howard Community College
Abigail Livingston, Howard Community College
Rohaan Mahmud, Howard Community College
Mingqian Huang, Howard Community College
Madhav Fadadu, Howard Community College
Kimia Samieniejad, Howard Community College
Mentored by: William Gretes, Ph.D.
Abstract
Larval Manduca sexta, commonly known as the tobacco hornworm, has a natural affinity for plants in the family Solanaceae. It has been previously demonstrated that this preference for specific host plants must be induced by prior feeding experience on those plants. However, it is not yet clear whether effects of these behavioral modifications are isolated to the rearing plant or extend to other closely related plants. Cohorts of larvae were reared on a single solanaceous plant (tobacco) or one of several non-solanaceous plants to the 5th instar, and later tested in an isolated, no-choice experiment. Survivability of the larvae (from 1st to 5th instar) in each cohort was recorded. To determine the palatability of a specific plant, data was gathered on how much leaf area the caterpillars consumed at hourly intervals. This information was then graphed as a percentage of the leaf remaining over time which was used to determine the rate of feeding.
Experimental results showed that there was an overall higher larval acceptance for tobacco regardless of their rearing history. The results obtained for larvae feeding on non-solanaceous plants were considerably more varied. Cabbage, for example, appeared surprisingly palatable across all rearing backgrounds (despite extremely low survivability on cabbage). Consumption of rapeseed and turnip varied more depending on diet history. Survivability was highest on tobacco, moderate on rapeseed and turnip and low in cabbage and soybean, where only a single larva for each survived to the fifth instar.
The survivorship data demonstrates that the nutritional value and general suitability of non-solanaceous plants varied greatly. The consumption data suggests that while the acceptance of tobacco was universally high and inflexible, the acceptance of non-solanaceous plants (even within the same family) were much more heterogeneous, with varying degrees of flexibility. Increases in the palatability of tobacco were therefore not observed. In contrast, some evidence of both inductions for the rearing plant and for other related plants were observed within the non-solanaceous plants tested. The latter would be the first such observation in the plant family Brassicae.
Introduction
Manduca sexta, more commonly known as the Tobacco Hornworm, is an insect that in nature primarily feeds on plants in nature within the one family of plants called Solanaceae. Insects that feed on a limited variety of food like Manduca sexta are refered to as oligophagous.
The family of plants preferred by the Tobacco Hornworm includes well-known plants like tomato, potato, tobacco, and other nightshades. The tobacco hornworm is known to eat certain plants outside of the solanaceous family. These include cowpea, soybean, and corkscrew vine from the family Fabaceae and rapeseed, turnip, and cabbage from the family Brassicae.
Some previous studies have shown that tobacco hornworms are willing to feed on, and can even be raised on, non-solanaceous plants [1, 2]. This raises the question as to whether these larvae are truly oligophagous. Nearly every behavioral study shows that the oligophagous nature of the tobacco hornworms can be influenced by the plant they were reared on [1,2,4,5]. When reared on a specific plant, larvae induce a preference towards that plant (induction of preference) or induce a preference towards other plants that are closely related to that reared plant (induction of oligophagy).
Yamamoto wanted to determine if Tobacco Hornworms were inherently oligophagous insects, as seen in nature, or if they were induced to oligophagy based on the plants they were reared on. To test this, he conducted an experiment under no-choice conditions: the insects were feed only one type of plant until they were first instar larvae. This included an array of solanaceous and non-solanaceous plants. The test concluded that first instar larvae ate a variety of non-solanaceous plants within 24 hours showing polyphagous characteristics. This supported the idea that Tobacco Hornworms were not born oligophagous but instead are polyphagous (able to feed on various plant species and families) at birth and become oligophagous (induction of oligophagy) when reared on solanaceous plants [1, 2].
Thus, behavioral patterns, even when increasing the variety of plants, is still consistent with Yamamoto’s results in which acceptable non-solanaceous reared larvae exhibit a loss of oligophagy that solanaceous reared larvae have and even induce a preference towards certain acceptable non-solanaceous plants. Although rearing might influence the strength of oligophagy and induce a preference, the reason for which Manduca sexta larvae strongly prefer solanaceous to begin with is still unknown.
del Campo and Renwick suggested that the host (solanaceous) preferability could be from the larvae becoming dependent on the chemical constituents of the solanaceous plants. Therefore, to induce a host specificity, the Tobacco Hornworms must become dependent on solanaceous chemical extracts [3]. To directly investigate the role of chemical constituents of the solanaceous plants on the induction of influence preferences, larvae were given a choice of a non-solanaceous disk (cowpea) treated with extract from a solanaceous plant (potato) or cowpea disk alone. The no-choice test showed that larvae reared on a solanaceous diet accepted any solanaceous foliage offered but rejected non-solanaceous foliage (cowpea). In contrast, larvae reared on a non-solanaceous diet equally accepted solanaceous and non-solanaceous foliage [3]. This further reinforced the idea that rearing influenced the strength of preferability because rearing on non-solanaceous foliage decreased the preferability for solanaceous foliage. The choice test showed that a much higher percentage of larvae reared on the solanaceous diet took their first bite on the cowpea disc treated with potato extracts than the control (cowpea). However, larvae reared on the non-solanaceous diet did not discriminate between the control disk and the cowpea treated with potato extract disk. Also, the percentage of larvae taking their first bite of a potato disk with cowpea extract was relatively the same regardless of their reared diet being solanaceous or non-solanaceous [3]. Therefore, the researchers concluded Manduca sexta larvae tend to be attracted to chemical constituents found in solanaceous foliage if reared on a solanaceous diet due to becoming dependent [3].
In this experiment, we aim to better understand feeding behavior (induction of preference and induction of oligophagy) and whether or not induction can cause increased feeding on the same plant species or related species. We also investigate hierarchy in food acceptability from consumption trials for various non-host (Brassicae and Fabaceae) and host (Solanaceae) plants.
Methods
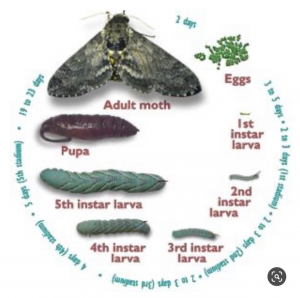
The greenhouse used to cultivate both plants and larvae was an environmentally controlled setting that maintained light, temperature, air, and water. The greenhouse had windows that permit UV light to reach the plants during the day and during the night provided multi-spectrum LED lights that emit a purple wavelength which enables plants to absorb the most amount of light. The greenhouse was also a temperature-controlled environment that consistently remained at 80℉, humidity was also maintained, and water was provided to plants regularly. Larval eggs were imported from Carolina Biological Supply ® by road. A group of five first instar larvae were isolated into clear plastic containers with a piece of foliage from a single plant species like Tobacco or one from either the Brassicaceae or Fabaceae family. Foliage was obtained by removing a healthy piece of leaf from a plant grown in the greenhouse. Then a piece of wet paper towel was wrapped around the stem to prevent the leaf from desiccation. The foliage was replaced every day and thoroughly cleaned. Enough leaves were provided to prevent competition among larvae for food. The survivorship was recorded daily noting the number of larvae alive, the number of larvae dead (removing the dead larvae in the process), the number of larvae reaching premolt, and the total number of larvae up until the fifth instar before testing.
Before reaching the fifth instar, larval premolts were isolated and placed into individual containers. Freshly molted fifth instars were later placed into an arena which was grounded by a plastic grid. The grid was painted and necessary for the larvae to have a matrix to grip to. A fresh leaf from either a member of the Brassicaceae or Fabaceae family, or a tobacco leaf was placed into the arena. The leaf was attached using a pin to prevent movement of the leaf during its consumption by larvae. The arena was covered by a petri dish. A calibration square was placed near each arena for analyzing data. Each arena was labelled including their test plant, the plant the larvae were fed on and the date when the experiment was conducted. A GoPro camera was placed on top of every arena (a total of 11 cameras) and took a picture at an interval of every 30 seconds over a 24-hour period (raw images). Adequate light was provided to avoid reflection.
The raw images from each experiment were collected and categorized into hourly images by selecting images at every hour. Hourly images were then uploaded to Adobe Photoshop where they were cropped with lasso and highlighted with the paint bucket feature. The portion of leaf remaining in every image was highlighted manually in a neon green shade (Analyzed images). The analyzed images were transferred to ELA (Easy Leaf Analysis application) to calculate the area of the leaf by each pixel. The software calculated the number of pixels in the leaf using variation in the ratio of red and blue light present. The pixels were then converted into cm2 to measure the area of the leaf consumed. After calculating the area, the data was used to create graphs which indicated the percentage of leaf consumed over a period of time (Results).
Results
Survivorship-
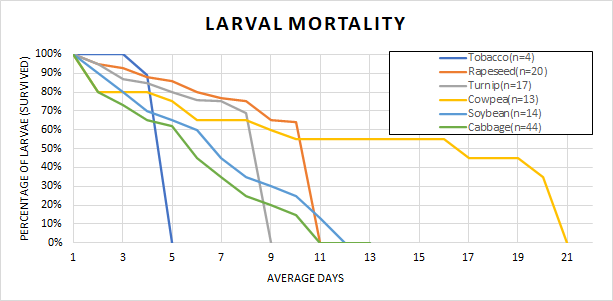
Survivorship results showed a noticeable difference in the rearing capabilities of the tested plants. Figure 1 shows the percentage of larval survivability over a given day combined of each replica of larvae that were tested. Tobacco-reared larvae had the lowest mortality rate with only 4 out of 35 (about 11%) larvae mortality rate. In contrast, cabbage-reared larvae and soybean-reared larvae had the highest mortality rate as 44 cabbage-reared larvae out of 45 died (99.98%) and 14 soybean-larvae out of 15 died (99.94%). Of the other two non-solanaceous plants we tested (rapeseed and turnip) showed high survival rates of 64% and 69% respectively (Figure 1).
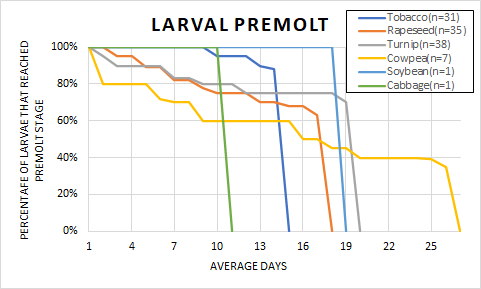
Figure 2 shows how long it took (in days) to reach the 5th instar for those larvae that survived. The single cabbage-reared larvae that survived took the fewest number of days to reach premolt (11 days). Cowpea-reared larvae took the largest number of days (26 days) for the 7 surviving larvae to reach premolt. The 31 suriving tobacco-reared larvae reached premolt on average in 14 days, which was the second fastest followed by rapeseed (18 days, n=35), soybean (19 days, n=1), and turnip (20 days, n=38). While it may appear that larvae reared on cabbage develop fastest, it should be noted that this data is based on the one surviving cabbage-reared lava, and is clearly an outlier that should not be used to make any conclusions.
Plant consumption-
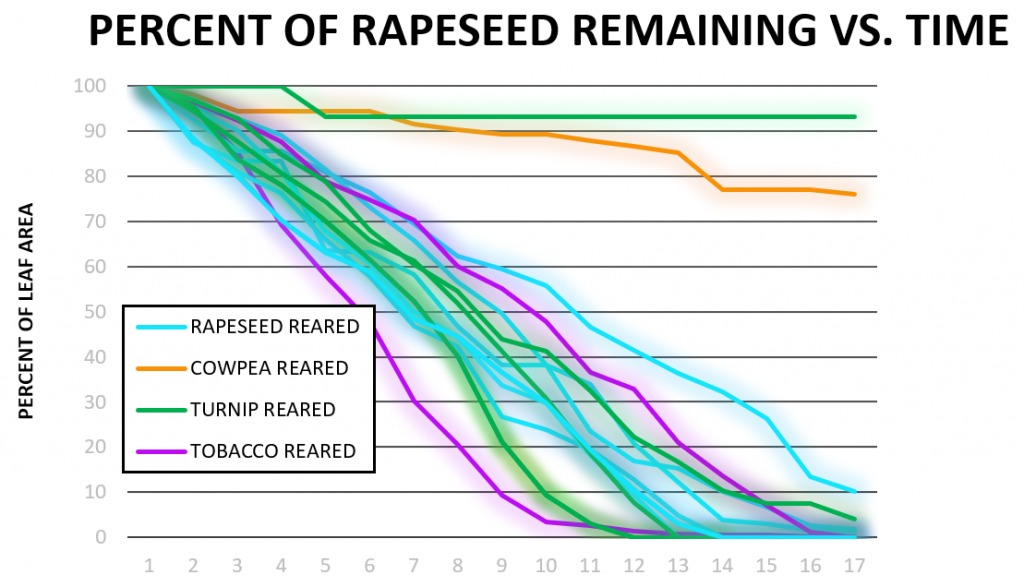
Larvae of different rearings were tested on one of the four plants and the resulting data were used to create figures 3a-d. Hourly increments of data were mapped for each cohort of larvae and separated by consumption on the plants (cabbage, rapeseed, tobacco, and turnip). A high consumption rate can be seen as a steep sloped line and low consumption rates were seen as flatter slopes. Data was collected for up to 24 hours. In some instances, the larvae stopped feeding prior to the 24-hour data collection period and did not fully consume the plant. For those data, the graph only shows the data collected during the time (15-18 hours) the larvae were still feeding. It was expected that larvae, no matter the rearing, would consume tobacco the fastest since it is a solanaceous plant and was seen to be highly palatable in previous research [2,4,5]. Consumption of tobacco was slightly faster on average than consumption of the other plants. However, there is not enough data to strongly draw conclusions due to the lack of larval rearings that were tested (Figure 3c). Since rapeseed is known as a very palatable non-solanaceous plant [5], we tested larvae raised on different plants including cowpea for their consumption of rapeseed. Surprisingly, rapeseed-reared larvae did not consume rapeseed the fastest: on average, both turnip-reared and tobacco-reared larvae consumed it faster (Figure 3a). A similar observation is shown up in Figure 3b, where the cabbage-reared larvae did not consume cabbage as fast as the larvae reared on other plants. There were two strong outliers in this graph: one turnip-reared larvae that stopped feeding at 93% and one cowpea-reared larvae that stopped feeding at 75% of the total leaf area (Figure 3a).
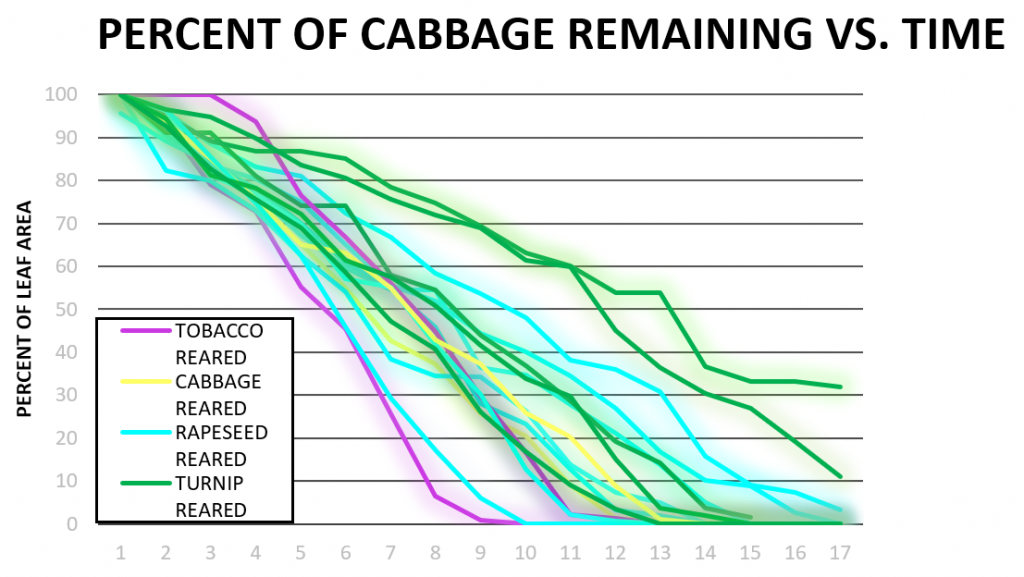
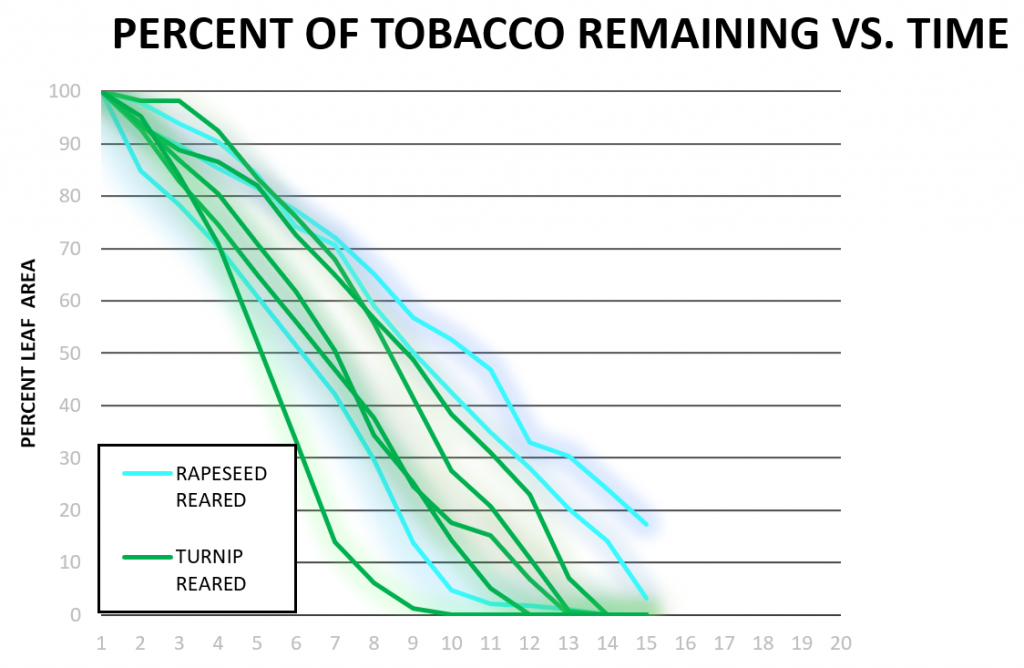
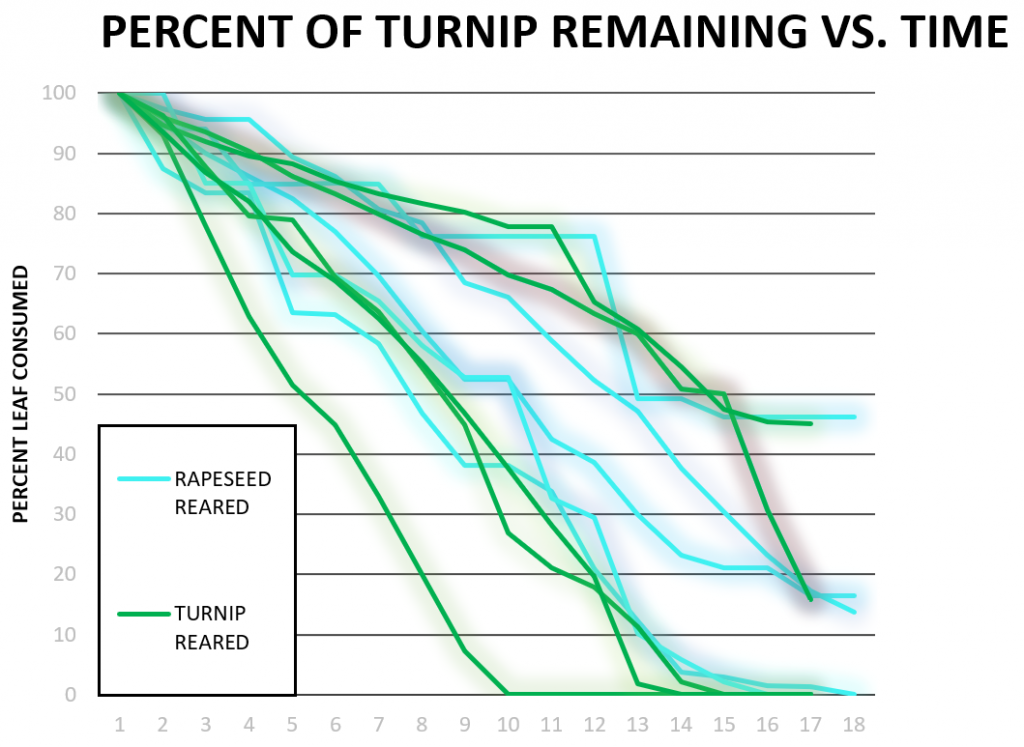
In Figure 3b, a trend similar to that shown in Figure 3a was observed. Cabbage-reared larvae did not consume cabbage the fastest: some rapeseed-reared, turnip-reared, and tobacco-reared larvae finished faster. Two cabbage-reared larvae were included in this Figure as the data from one cabbage-reared larvae was pulled from data obtained during similar experiments performed in our laboratory in 2018 and analyzed using our updated procedures. Figure 3c shows that tobacco was consumed at a faster both rapeseed-reared and turnip-reared larvae, which is consistent with previous observations. One rapeseed larva did not finish within 15 hours during these trials which was the only outlier in this Figure. Consumption of turnip vs. time (Figure 3d) showed the only evidence of induction of preference, where a turnip-reared larva finished a wide margin faster than other caterpillars on rapeseed. Overall tobacco was appeared to be the fastest-consumed plant (Figure 3c), even the larvae reared on turnip or rapeseed.
Discussion
Increasing Resolution
Here, we describe our efforts to better understand feeding behavior of Manduca sexta by making significant improvements in the methods of data collection and data mapping. Major problems that were previously faced included the data load and difficulties in the software. ELA requires specific ranges of color ratios, something that needs to be precisely customized for each leaf so that the software can differentiate between the leaf and the background, and between the leaf and the caterpillar. Due to lighting, glare, and the fact that the caterpillars and the leaves are very similar in color,, finding the right setting was very difficult and often resulted in a compromise which resulted in regions of the leaf being left out, or the caterpillar being partially covered— incorrectly registering pixels.
To improve the fidelity of our experiments and further prevent misrepresentation of the data, we developed a new method of data collection. Our current method involves using the XX in Adobe Photoshop to manually selected hourly images of the leaf. This approach provided a precise mapping of the leaf area with a single bright green color that ELA can easily differentiate. This color overpowered all the glare and worked with the auto pixel tool, so we did not have to adjust the sliders for each batch of images. Another benefit of this approach was that it allowed us to correctly map data in circumstances where the caterpillar covered parts of the leaf that was already consumed. Using the Adobe Photoshop, we can ould interpret the part of the leaf the larvae consumed, and then add color over the caterpillar— something that previously was just left misrepresented. This technique did add additional work to our data analysis period, but additional improvements were made to the previous procedure, which relived the overall workload. To select data points, the previous group first cropped the thousand (or more) pictures collected from the individual trial, and then imported those images into ELA while adjusting sliders and making sure the images were mostly accurate. This process took hours for the system to process alone, taking up many gigabites of space o the computer, and was so much time-consuming that they weren’t able to thoroughly analyze the data using ELA. By only analyzing 24 hourly images, we were able to analyze all the data collected since this approach saved hours of work, hard drive space, and had fewer images that needed to be analyzed.
Challenges with our current experiments primarily resulted from the survival of our larvae from certain rearings (for example soybean) and limitations when trying to grow particular plants species (for example corkscrew vine). Through the duration of these experiments, we had only 1 out of the 45 larave raised on cabbage larvae and only 1 out of the 15 larvae raised on soybean survive long enough to be tested for feeding preferences. Rearing more larvae to the 5th instar on these two plants would have allowed us to obtain more data from which we could then make conclusions.
Another group of larvae that we would have liked to see in our results more was tobacco-reared larvae. Although we raised many tobacco-reared larvae to the 5th instar and tested them on every plant, we were not able to analyze these data in enough time for it to be included in this paper. Corkscrew vine was a plant that we would have liked to test, but shipments of seeds did not make it to our laboratory in time for them to be tested.
Future plans include continuing to collect larval consumption data, especially from larvae reared on the less suitable plants (i.e., soybean and cabbage). We would also like to test a wide variety of solanaceous and non-solanaceous plants that have been examined in previous studies in order to determinin preference hierarchies and strengthening induction claims. Improvements to the data collection pipeline can now allow future groups to spend more time collecting larval data, leading to a bigger study.
Host plant suitability
The survivorship data (Figure 1) shows that certain plants are more suitable for larval development than others. Solanaceous tobacco yielded the highest survivability at 89%, which was expected due to the hornworms’ natural inclination to feed on tobacco plants. There was a great variation in the levels of host plant survivability of larvae on non-solanaceous plants. Specifically, larvae raised on rapeseed and turnip showed increased survivability compared to cabbage, soybean, and cowpea-reared larvae, which had fewer than 20% of the larvae reach the 5th instar. These results are somewhat unexpected because cabbage is in the same family (Brassicaceae) as turnip and rapeseed. However, only one larva reared on cabbage made it to the 5th instar (out of 44) and therefore would be tested, so it is not yet clear whether or not this is a real difference. If true, this result could suggest that there are subtle differences between the plants in either composition or nutrition that causes change in survivability.
Test plant acceptability
In our consumption data, determining if there is any induction of preference or induction of oligophagy we were able to observe several trends. The data obtained from larvae tested on rapeseed (Figure 3a) had two outliers (turnip-reared and cowpea-reared), but the rest of the larvae all finished rapeseed plants around the same time. These data conflict with our original hypothesis that rapeseed-reared larvae would finish rapeseed faster than larvae raised on other plants (induction of preference). Figure 3b shows similar trends where cabbage-reared larvae did not finish significantly faster than other rearings of larvae and were surprisingly slower than tobacco, rapeseed, and even some turnip-reared larvae. This data also rejects our hypothesis that the cabbage-reared larvae would consume the fastest. In addition, it is not clear whether any induction of oligophagy occurred since larvae raised on a solanaceous plant consumed cabbage faster than larvae reared on the same family of plant (turnip and rapeseed). Tobacco was seen to be a very palatable plant for larvae despite their rearing (Figure 3c) and no consumption difference was seen between turnip or rapeseed-reared larvae when tested on tobacco.
The only evidence of induction of preference, shown in Figure 3d, was when turnip-reared larvae consumed turnip faster than rapeseed-reared larvae. This was seen in only one larva and when the data is averaged, the difference between the two rearings of larval consumption is insignificant and almost identical. The previous study [4], conducted a similar test using turnip-reared larvae tested on turnip and discovered that turnip-reared larvae consumed turnip slower than they do on other plants like tomato (solanaceous) and rapeseed (non-solanaceous). Thus, no induction of feeding preference was observed in this dataset. Additional experiments will be required to determine whether feeding preferences are induced or not.
Conclusions
Overall, the survivorship data reported in this study provides strong evidence that certain plants are much more suitable for rearing Manduca Sexta larvae than others. In addition, the consumption rate data presented here indicates that certain plants are much more palatable for tobacco hornworms than other plants.
The consumption rates for non-solanaceous plants (turnip and rapeseed) were higher than expected. Interestingly, these highly palatable non-solanaceous plants all belong to the same family of plants: Brassicae. More data are required to examine the palatability of other non-solanaceous families like Fabaceae (cowpea and soybean). Previous studies, along with our research, suggests that there are underlying mechanisms affecting feeding preferences in hornworms. Better characterization of these mechanisms requires many additional studies and combinations of controlled research. Finally, this study provides major improvements in the protocol for measuring leaf consumption involving improvement of the fidelity of measurement when using ELA— something that can greatly improve research on this topic in the future.
Acknowledgements
This material is based upon work partially supported by the National Science Foundation under Grant No. 1458149.
Contacts: hcarlson@howardcc.edu, Abigail.livingston@howardcc.edu, Mingquian.huang@howardcc.edu, Rohaan.mahmud@howardcc.edu, Mahdav.fadadu@howardcc.edu Kimia.samieniejad@howardcc.edu, wgretes@howardcc.edu
References
[1] R. T. Yamamoto, “Induction of Hostplant Specificity in the Tobacco Hornworm, Manduca Sexta”, F. Insect Physiology, vol. 20, pp. 641-650, 1974.
[2] G. de Boer and F. E. Hanson, “Foodplant selection and induction of feeding preference among host and non-host plants in larvae of the tobacco hornworm Manduca sexta,” Entomologia Experimentalis et Applicata, pp. 177-193, 1984.
[3] M. L. del Campo and A. A. Renwick, “Dependence on host constituents controlling food acceptance by Manduca sexta larvae,” Entomologica Experimentalis et Applicata, pp. 209-215, 1999.
[4] C. Frimpong, R. C. Roberts, T. R. Gongs, A. Faeque and W. C. Gretes, “Feeding Inductions on Non-solanaceous Plants in Larval Manduca sexta,” Journal of Research in Progress, pp. 74-83, 2018.
[5] W. C. Gretes, E. A. Stanwyck and F. E. Hanson, “Innate and acquired components of oligophagy in the herbivorous lepidopteran, Manduca sexta,” Entomological Experimentalis et Applicata, pp. 259-271, 2016.
[6] C. Berkley, “Teach Life Cycles with the Tobacco Hornworm”.Carolina Biological Supply Company.
[7] The Worm Lady. 2013. http://thewormlady.ca/hornworm-life-cycle.php.