Salivary Alpha-Amylase and the Effects of Legume-based Inhibitors
Abdelrahman Abdelaziz , Howard Community College
Ayushi Dave, Howard Community College
Roshae Roberts, Howard Community College
Mentored by Hannah Pie, Ph.D.
Abstract
Alpha-amylase is an essential digestive enzyme found in humans. In our bodies, it is responsible for starch hydrolysis into dextrins and eventually maltose within saliva and the small intestines. Maltase then hydrolyzes maltose into glucose that can be utilized by the cells. The functionality and reaction rate of alpha-amylase like all other enzymes is related to pH, temperature and initial concentration of substrate. In the body, an additional factor to consider is the presence of inhibitors that prevent enzymes from binding with their substrates. Various legumes have been shown to include compounds that inhibit alpha-amylase activity by preventing the hydrolysis of starch and limiting the release of glucose. The goal of this study was to identify the inhibiting activity of various legumes on human salivary alpha-amylase utilizing a starch-iodine detection test. The inhibitory compounds of the following legumes: black beans, kidney beans, black eyed beans and white beans, were extracted using ethanol fractionation and tested to determine which legume has the most inhibitory effect. Our results showed that kidney beans had the highest imbibition activity on salivary alpha-amylase. This was followed by black beans, white beans and black-eyed peas, respectively. The fact that kidney beans display strong inhibition on salivary alpha-amylase could make them a strong candidate for possibly replacing diet pills as a healthier alternative with reduced side effects.
Introduction
Carbohydrates, lipids, proteins and nucleic acids are the four basic macromolecules found in the foods we ingest. These molecules need to be broken down further into their monomers in order to be absorbed into the cells of gut lumen. This is done primarily through the chemical action of water and hydrolytic digestive enzymes. Each macromolecule has different hydrolytic enzymes that cleave the bonds between its monomers. Amylase, produced within saliva and the pancreas, is the hydrolytic enzyme responsible for the hydrolysis of starch. Starch is a polysaccharide comprised of thousands of glucose monomers. Digestion of starch begins with the actions of salivary alpha-amylase to break down starch into dextrins (shorter polysaccharides) and then to maltose (a disaccharide). To further break it down, the enzyme maltase hydrolyzes it to glucose.
The amount of alpha-amylase produced and the number of gene copies that express alpha-amylase (AMY1) varies among individuals [2] The ability of some people to have multiple copies of AMY1 is thought to have evolved as populations increased starch intake within their diet [2]. Populations with diets rich in starch such as rice and potatoes correlate to more copies of AMY1 and higher levels of alpha amylase activity [3]. Inversely, populations with diets low in starch have less copies of AMY1 and lower levels of alpha-amylase activity [3].
Various legumes from the Fabaceae family have been shown to include compounds that inhibit alpha-amylase activity by preventing the hydrolysis of starch and limiting the release of glucose [4]. Phaseolus vulgaris subspecies beans, such as kidney beans, black beans, great northern white beans, have shown to exhibit moderate to high levels of pancreatic alpha-amylase inhibitory activity [4]. In contrast, the black-eyed peas, a subspecies of the cowpea Vigna unguiculata, displayed lower inhibition activity for pancreatic alpha-amylase [1]. While pancreatic alpha-amylase inhibition by these beans has been examined before, salivary alpha-amylase has not, so by conducting this study we are contributing to current literature. Current research is focusing on the use of beans and their inhibitory compounds for anti-obesity and diabetic treatments.
This study sought to examine our own salivary alpha-amylase activity to affirm the correlation between diet and concentration. All students expected to exhibit a moderate to high amount of alpha-amylase as all had diets rich in starch. Additionally, this study sought to examine the inhibiting activity of various legumes on human salivary alpha-amylase utilizing a starch-iodine detection test. The inhibitory compounds of the Phaseolus vulgaris beans (black beans, kidney beans, great northern white beans) and the Vigna unguiculata subsp.bean (black-eyed peas) were extracted using ethanol fractionation and tested to determine which has the most inhibitory effect. Based on previous research in human pancreatic alpha-amylase, we expect kidney beans to have the highest inhibitory effect on human salivary alpha-amylase.
Methods and Materials:
Procedure for Individual Salivary Alpha-Amylase Starch-Iodine Assay [4]:
In order to test how fast one’s alpha amylase breaks down carbohydrates like starch, alone and in the presence of inhibitory compounds from the beans, we used the starch iodine test. During these tests we maintained a constant temperature and pH to maintain a controlled environment to test for the functionality of the enzyme. In order to quantitatively analyze the absorbance of the starch hydrolysis, we used a spectrophotometer. This allowed us to quantify the presence of starch numerically, which indicates how much enzyme was present.
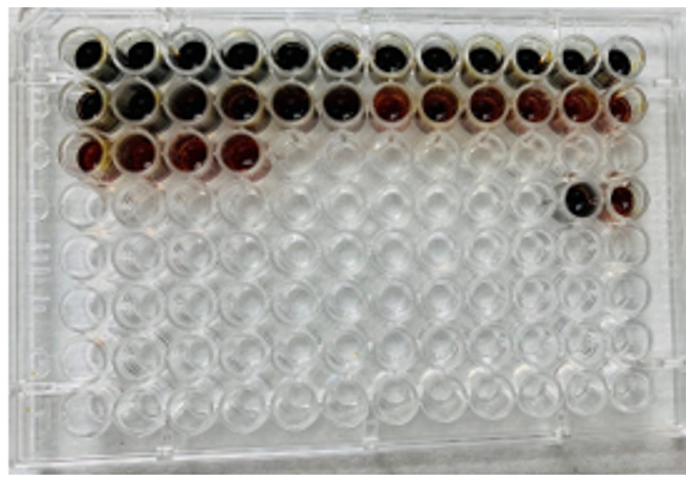
We started by preparing 96-well plates containing 50 μL Lugol’s iodine and 10 μL 0.1 M HCl in each well to stop the reaction and for visible detection of starch. Each student collected 2 ml of saliva for testing and then prepared a 10% dilution of the saliva in NaCl (pH 7.0). Testing solutions of 5.0 mL of 1% starch and 4.0 mL of NaCl (pH 7.0) were prepared, then 1 mL of the 10% saliva was added to start the reaction. Every 30 seconds, 50 ul of testing solution was added to a well to stop the reaction until a visible achromatic point (the point at which all the starch had been completely hydrolyzed to maltose) was reached (Fig 1.). A positive control was used to show what the color looks like in the presence of starch with no enzyme, while a negative control was used to see the color of the iodine test with the presence of no starch or completely hydrolyzed starch. Spectrophotometric analysis at 595 nm was conducted to determine the achromatic point at which all the starch has been hydrolyzed using the negative control as the blank.
Procedure for Salivary Alpha-Amylase Standard Curve:
The starch-iodine assay was used for determining the achromatic points of multiple concentrations of a standard human salivary alpha-amylase (10, 25, 50, 75, 100, 125 U/mg alpha amylase) to produce a standard curve (Fig 3.). The human salivary alpha-amylase used for the standard was purchased from Sigma-Aldrich (Sigma-A0521).
Procedure for Preparation of Legume Inhibitors [1]:
Dried legumes (kidney beans, black beans, great northern white beans, and black-eyed peas) were purchased from a local market. 4.0 g of each legume was ground using a mortar and pestle to a fine meal. 25 mL of 10 mM PB buffer (7 pH) was added and solutions stirred for 24 hours at 4°C. Solutions were centrifuged for 30 minutes at 8,500 rpm and then 1 hour at 5,000 rpm. The inhibitory compounds were extracted with 48% v/v ethanol (Fig 2.) and centrifugate for 1 hour at 5000 rpm. The supernatant containing the inhibiting compounds was removed and used for the inhibition assay.
Procedure for Testing Inhibition of Alpha-Amylase by Legumes:
The starch-iodine assay was used for determining the achromatic points of the four extracted inhibitors in the presence of 40 U/mg human salivary alpha-amylase.

Results
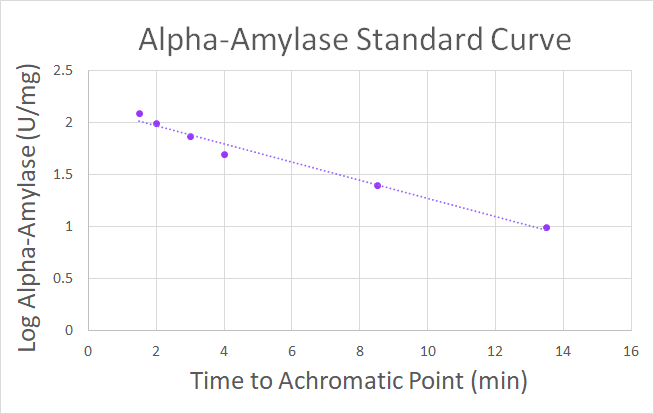
The standard curve (Fig 3.) shows that there is an inverse relationship between enzyme concentration and time it takes to reach the achromatic point. The quicker the reaction takes to reach its achromatic point, the higher the concentration of enzyme.
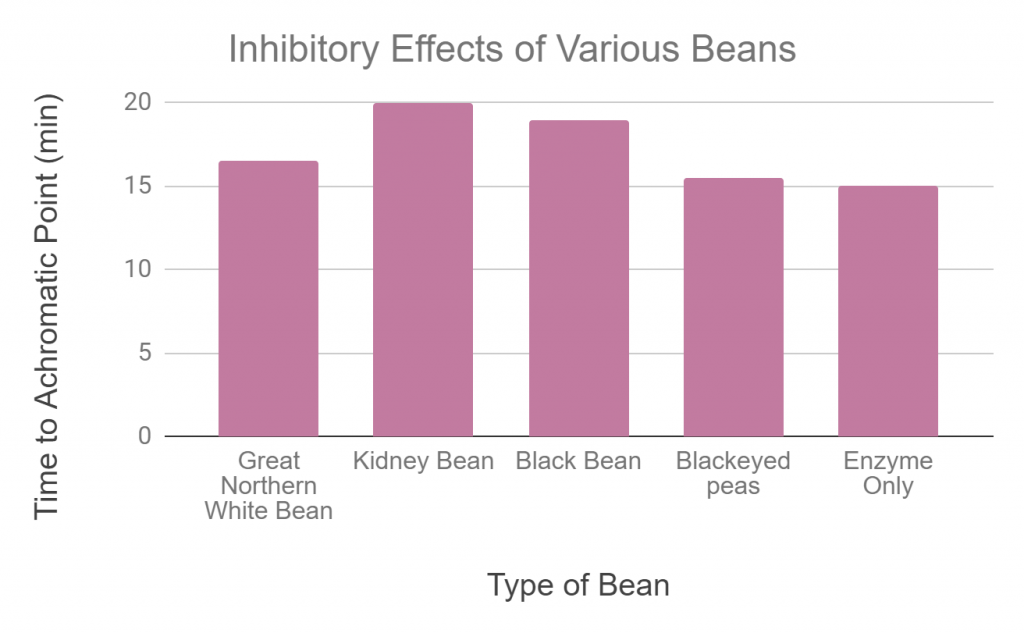
The legume reactions reveal that some of the beans have more inhibitory effects than others (Fig 4.). The higher the inhibitory effect, the lower the enzymatic activity. Kidney beans took the longest time to reach achromatic point thus displaying the highest inhibition (lowest enzymatic activity) on alpha-amylase relative to other beans tested. Black-eyed peas have the most similar achromatic time to the enzyme only negative control indicating a low inhibitory effect (high enzymatic activity). Black beans show the second most inhibitory effect (second lowest enzymatic activity) with great northern white beans following.
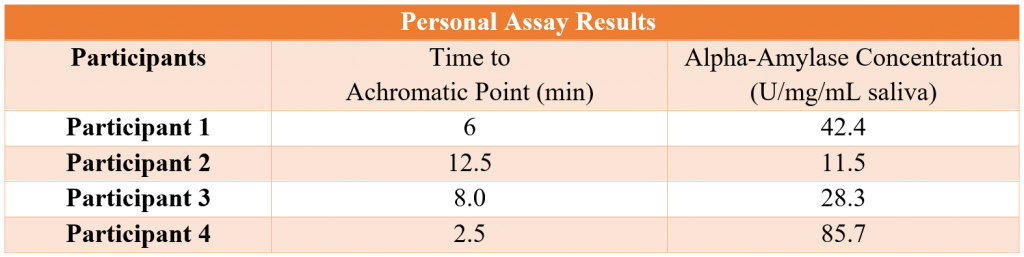
Both participant 1 and participant 4 had an alpha-amylase concentration of 42.4 and 85.7 U/mg/mL saliva, respectively. However, participants 1 and 2 had an alpha-amylase concentration of 11.5 and 28.3 U/mg/mL saliva, respectively. While all the participants had alpha amylase concentrations much lower than expected, there are other factors besides diet which contribute to the number of alpha-amylase copies and overall amount of the enzyme produced in the saliva.
Discussion and Conclusions
Our results indicate that kidney beans had the highest inhibition followed by black beans, white beans and finally black-eyed peas. This means that at the same concentration of alpha-amylase, kidney beans will inhibit the activity of the enzyme the most; as evidenced by the fact that they took the longest to reach the achromatic point. Out of all the beans tested from the Fabaceae family, black eyed peas were the only one from the genus Vigna, while the other three were from the genus Phaseolus. This possibly explains why black-eyed peas had the least inhibition activity. Our results also indicate that the best candidates for bean-based diet pills are those that are subspecies of Phaseolus vulgaris as beans from this species were the most efficient and blocking the activity of alpha-amylase. These results were previously supported by a study [5] which showed that an alternative to low glycemic index diet could be products which slow down the rate at which carbohydrates are absorbed. This is done by inhibiting alpha-amylase and glucosidase activity. In beans that are subspecies of Phaseolus vulgaris, specifically great white kidney beans, a compound known as Phase 2 Carb Controller has shown the ability to cause weight loss with doses ranging from 500-3000 mg per day [5]. Besides the potential of Phase 2 as a weight loss product, it also showed the ability to reduce post-prandial spike in blood glucose levels [5]. All these benefits are due to the ability of Phase 2 to block the activity of alpha-amylase which brings about the possibility of identifying more efficient alpha-amylase inhibiting compounds in beans of the same or other families.
For the assay testing the concentration of alpha-amylase in our four participants, the results for both participant 2 and participant 3 were unexpected. All four of the participants come from areas that consume high amounts of starch and legumes. So, the expected result was that they would all have multiple copies of the alpha-amylase gene, which would positively correlate with the amount of alpha-amylase in their saliva. The effect of consuming large amounts of legumes on the alpha-amylase concentration in individuals is still unclear, and there might be many other contributing factors effecting the number of alpha-amylase gene copies or expression of those genes within individuals. The known range of salivary alpha-amylase activity is 1-371 U/mL saliva with an average of 93 U/ml (±62), so all participants displayed below average salivary alpha-amylase concentrations [1].
Our next steps will be to examine factors that affect the activity of alpha-amylase in our saliva to have a better understanding of the effect inhibitors have on that activity . Furthermore, we plan to test how well diet pills inhibit the activity of salivary alpha-amylase to know whether they are effective or not. By testing different kinds of beans and comparing their inhibition activity to those of diet pills, we would possibly be able to provide better alternatives.
Acknowledgements
Thank you for the STEM Scholars Program for providing us with the opportunity to do this project, Dr. Hannah Pie for her wonderful guidance, patience and time commitment, and to Professor Darling for providing the supplies needed for the experiments.
Contacts: abdelrahman.abdelaziz@howardcc.edu, adave6283@howardcc.edu, roshae.roberts@howardcc.edu, hpie@howardcc.edu
References
[1] Ghorbani, F., Sadeghi, M., Aghaei, A., Ghahghaei, A., Jalili, C., & Khodarahmi, R. (2018). Study of α-Amylase Inhibitors among Different Bean Cultivars and Evaluation of their Effectiveness Compared with a Commercial Product using In Vitro/In Vivo Experimental Systems. Journal of Research in Medical and Dental Science,6(1), 381-391. doi:10.5455/jrmds.20186162
[2] Mandel, A. L., des Gachons, C. P., Plank, K. L., Alarcon, S., & Breslin, P. A. (2010). Individual differences in AMY1 gene copy number, salivary α-amylase levels, and the perception of oral starch. PloS one, 5(10).
[3] Perry, G. H., Dominy, N. J., Claw, K. G., Lee, A. S., Fiegler, H., Redon, R., … Stone, A. C. (2007). Diet and the evolution of human amylase gene copy number variation. Nature Genetics, 39(10), 1256–1260. http://doi.org/10.1038/ng2123
[4] Xiao, Z., Storms, R., & Tsang, A. (2007). Corrigendum to “A quantitative starch–iodine method for measuring alpha-amylase and glucoamylase activities” [Anal. Biochem. 351 (2006) 146–148]. Analytical Biochemistry,362(1), 154. doi:10.1016/j.ab.2006.12.021
[5] Barrett, Marilyn L, and Jay K Udani. “A proprietary alpha-amylase inhibitor from white bean (Phaseolus vulgaris): a review of clinical studies on weight loss and glycemic control.” Nutrition journal vol. 10 24. 17 Mar. 2011, doi:10.1186/1475-2891-10-24